
Concept explainers
a.
Interpretation:
Whether
Concept Introduction:
We can predict the shape of a particular molecule by the knowledge of their
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
a.
Answer to Problem 7.67PAE
Solution:
The geometry of
Explanation of Solution
The electronic configuration of Xeis
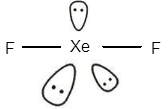
The geometry of
The central atom has three lone pairs of electrons
b.
Interpretation:
Whether
Concept Introduction:
We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory. According to this theory, the atoms take such a position in which there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any. For Linear Molecular geometry, the molecules of an atom are arranged in a straight line making an angle of 180o with the lone pairs if any. A lonepair is the pair of an electron occupying the orbital but not taking part in the bonding.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
b.
Explanation of Solution
The geometry of
is linear and it has 0 lone pairs of an electron on the central atom The electronic configuration of C is
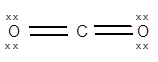
The geometry of
The central atom has no lone pair of electrons
c.
Interpretation:
Whether
Concept Introduction We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory. According to this theory, the atoms take such a position in which there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any. For Linear Molecular geometry, the molecules of an atom are arranged in a straight line making an angle of 180o with the lone pairs if any. A lonepair is the pair of an electron occupying the orbital but not taking part in the bonding.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
c.
Answer to Problem 7.67PAE
lp-lp>lp-bp>bp-bp
Solution: The geometry of
Explanation of Solution
The electronic configuration of Be is
Structure of
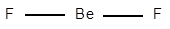
The geometry of
The central atom has no lone pair of electrons
d.
Interpretation:
Whether
Concept Introduction We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory. According to this theory, the atoms take such a position in which there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any. For Linear Molecular geometry, the molecules of an atom are arranged in a straight line making an angle of 180o with the lone pairs if any. A lonepair is the pair of an electron occupying the orbital but not taking part in the bonding.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
d.
Answer to Problem 7.67PAE
Solution:
The geometry of
Explanation of Solution
The electronic configuration of O is
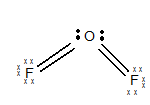
The geometry of
The central atom has two lone pairs of electrons
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry for Engineering Students
- 7.56 Draw the Lewis dot structures of the following compounds and identify the number of pi bonds in each. (a) Cl2O , (b) H2CCH2 , (c) HCCCN , (d) SiO2arrow_forwardHow many and bonds are present in the molecule HCN?arrow_forward7.55 Draw the Lewis dot structure of the following species and identify the number of pi bonds in each. (a) CS2 , (b) CH3Cl , (c) NO2 , (d) SO2arrow_forward
- In each of the following molecules, a central atom is surrounded by a total of three atoms or unshared electron pairs: SnCl2, BCl3, SO2. In which of these molecules would you expect the bond angle to be less than 120? Explain your reasoning.arrow_forward7.52 How does orbital overlap explain the buildup of electron density between nuclei in a chemical bond?arrow_forward2.5 Which of the following bonds are nonpolararrow_forward
- Which species has the smaller bond angle, H3O + or H2O? Explain?arrow_forward6.) The total number of valence electrons for the molecule NH2OH is ________.arrow_forwardWhy will C2H6N not fulfill the octet rule since it exceeds the maximum number of Carbon and Nitrogen bonds? Why can't C2H6N molecules exist?arrow_forward
- Identify which of these Lewis structures (A, B, C or D) is correct for NOF and what is the VSEPR shape of the compound?arrow_forward4.Examine the model and real structures for SO2. A.In what way does the central atom violate the octet rule? When is it possible for molecules to have this exception to the octet rule? B.Calculate the difference in the bond angle between the real and model structure? Is this difference larger or smaller than the difference observed for H2O? Why is the deviation between the real and model structure different for SO2compared to H2O? The deviation for H2O is 5 degreesarrow_forward6 Electronic geometry is the three-dimensional shape of molecules. True or false?arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning




