
Interpretation:
The value of reverse reaction rate constant
Concept Introduction:
The
For a general equilibrium reaction as follows:
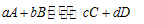
The expression for equilibrium constant of the recation can be written as follows:
Here,
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Introduction to General, Organic and Biochemistry
- 7-64 As we shall see in Chapter 20, there are two forms of glucose, designated alpha and betawhich are in equilibrium in aqueous solution. The equilibrium constant for the reaction is 1.5 at 30°C. (a) If you begin with a fresh 1.0 M solution of D-glucose in water, what will be its concentration when equilibrium is reached? (b) Calculate the percentage of glucose and of glucose present at equilibrium in aqueous solution at 30°C.arrow_forward7-29 The following reaction was allowed to reach equilibrium at 25°C. Under each component is its equilibrium concentration. Calculate the equilibrium constant, K, for this reaction.arrow_forwardIn Chapter 3, we discussed the conversion of biomass into biofuels. One important area of research associated with biofuels is the identification and development of suitable catalysts to increase the rate at which fuels can be produced. Do a web search to find an article describing biofuel catalysts. Then, write one or two sentences describing the reactions being catalyzed, and identify the catalyst as homogeneous or heterogeneous.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





