
(a)
Interpretation:
The major product of the reaction has to be identified.
Concept introduction:
Carbocation: Carbocation is a positive charged species and vital intermediate in
Hydrobromination:
A hydrobromination reaction is one of the electrophilic additions to
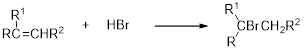
(b)
Interpretation:
The major product of the reaction has to be identified.
Concept introduction:
Carbocation: Carbocation is a positive charged species and vital intermediate in organic synthesis and its movement depends on the stability of the intermediate and the product formation.
Hydrobromination:
A hydrobromination reaction is one of the electrophilic additions to alkenes to yield the corresponding bromo alkanes. In this reaction the bromine atom adds to the double bond carbon which is having lesser number of hydrogen or more substituted carbon (Markovnikov's rule).
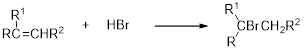
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Essential Organic Chemistry (3rd Edition)
- Draw the products obtained in each of the following reactions, assuming that only one equivalent of each reagent is used in each case:arrow_forwardWhich product will be formed in the highest yield from the following reaction?arrow_forwardFill in the missing reagent/product(s) for the following reactions.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
