
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
6th Edition
ISBN: 9781305687875
Author: Gilbert
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 7.3, Problem 1E
Interpretation Introduction
Interpretation:
The exclusion of thermal charge of pi bond needs to be determined as the starting step for the isomerization of 9 to 10.
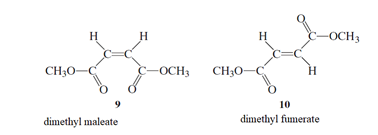
Concept Introduction :
Stereoisomers are the isomers with different spatial arrangement of the atoms, instead of order of atomic connectivity. It has the same molecular formula and the similar connectivity except for the procedure within 2D or 3D space. Like cis- and trans-but-2-ene both have two CH3 groups 2-H and a C=C but connectivity is different in the space.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using fishhook arrows to propose an initiation step for the reaction. Light (hν) induces homolytic cleavage of a weak bond. The Sn-H bond is rather weak and is therefore highly susceptible to homolytic cleavage.
Define mechanism for LiAlH4 Reduction of RCHO and R2C=O
Given the mechanism of the Heck reaction, illustrate why the Suzuki coupling of bromoethane and Ph-B(OH)2 might be challenging.
Chapter 7 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
Ch. 7.2 - Prob. 1ECh. 7.2 - Prob. 2ECh. 7.2 - Prob. 3ECh. 7.2 - Prob. 4ECh. 7.2 - Prob. 5ECh. 7.2 - Prob. 6ECh. 7.2 - Prob. 7ECh. 7.2 - Prob. 8ECh. 7.2 - Prob. 9ECh. 7.2 - Prob. 10E
Ch. 7.3 - Prob. 1ECh. 7.3 - Prob. 2ECh. 7.3 - Prob. 3ECh. 7.3 - Prob. 4ECh. 7.3 - Prob. 5ECh. 7.3 - Prob. 6ECh. 7.3 - Prob. 7ECh. 7.3 - Prob. 8ECh. 7.4 - Prob. 1ECh. 7.4 - Prob. 2ECh. 7.4 - Prob. 3ECh. 7.4 - Prob. 4ECh. 7.4 - Prob. 5ECh. 7.4 - Prob. 6ECh. 7.4 - Prob. 7ECh. 7.4 - Prob. 8ECh. 7.4 - Prob. 9ECh. 7.4 - Prob. 10ECh. 7.4 - Prob. 11ECh. 7.4 - Prob. 12ECh. 7.4 - Prob. 13ECh. 7.6 - Prob. 1ECh. 7.6 - Prob. 2ECh. 7.6 - Prob. 3ECh. 7.6 - Prob. 4ECh. 7.6 - Prob. 5ECh. 7.6 - Prob. 6ECh. 7.6 - Prob. 7ECh. 7.6 - Prob. 8ECh. 7.6 - Prob. 9ECh. 7.6 - Prob. 10E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The E1 mechanism (unimolecular elimination) of Elimination ?arrow_forwardAt step 4 in the mechanism of structure 5, at what does the arrow point?arrow_forwardShow the detailed reaction mechanism (Suzuki Cross-Coupling Reaction) of benzo[b]quinolizinium-9-trifluoroborate and 9-arylbenzo[b]quinolizinium derivativesarrow_forward
- What are the fragment ions obtained for the following through both the one bond and 2 bonds fragmentation pathways a) (2R, 5S) 5-methyl-2-heptanol b) benzyl isobutly ether c) (CH3)3CCH2COCH(CH3)CH2CH3 d) (3E, 5Z)-2,5,8,9-tetramethyldodec-3,5-dienearrow_forwardUseful Information: sodium metam: Commercial grade sodium metam is 33% pure sodium metam by weight and has a density of 1.2 g/mL. Hydrolysis of metam (Methyl isothiocynate) + H2S Rate constants for hydrolysis for metam (base catalyzed hydrolysis is negligible) = 300 mol -1 L. sec -1 , = 1 x 10-8 sec-1 Metam also undergoes photolysis to methyl isothiocyanate with a half-life of 1.6 hrs. Data for Sacramento River discharge Q = 75,000 L/min mean depth = 0.30 m pH = 7.8 mean width = 3.2 m dispersion coefficient D = 1.6 x 102 m2/min Assuming the spill acts acts as a single point source. How long does it take for the maximum contaminant concentration to get to Lake Shasta? If Na+ acts as conservative (i.e., it is not transformed) tracer, calculate the maximum concentration (in g/L) at Lake Shasta. (Hint: remember that sodium only constitutes a…arrow_forwardwhat type of mechanisims is needed for this?arrow_forward
- Propose a reaction mechanism for P4S3 + I2 isomerizationarrow_forwardDefine the features of E1 Mechanism ?arrow_forwardQ1: Give one lysis buffer that is commonly used for western blotting experiments and include its components Q3: To make sure that you used a similar amount of samples, what important step should be done before proceeding the electrophoresis stage? Q4. Why is it necessary to store the prepared lysates in a very low temperature?arrow_forward
- At the start of lab, Anthony adds vanillin to his Erlenmeyer flask, then adds sodium hydroxide, and lets the suspension cool. i. With minimal words, identify the reaction that takes place between sodium hydroxide and vanillin. ii. Is sodium borohydride capable of inducing the same reaction as (i)?arrow_forwardQuestion: How do quantum mechanical effects influence the stability and reactivity of molecules with non-classical carbocations, such as the 2-norbornyl cation, and how does this impact the reaction mechanisms and outcomes?arrow_forwardCarbonic anhydrase catalyzes the hydration of carbon dioxide: H2O + CO2 -> HCO3- + H+The following data is available: (image) - Calculate the Michaelis Menten constants - In the presence of sulfanilamide the Michaelis Menten constants for the reaction are Vmax: 7.57e-5M/s and Km: 20M. What type of compound is sulfanilamide and what is its mechanism of action?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
SAR of Anticancer(Antineoplastic) Drug/ Alkylating agents/ Nitrogen Mustard; Author: Pharmacy Lectures;https://www.youtube.com/watch?v=zrzyK3LhUXs;License: Standard YouTube License, CC-BY