
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.6, Problem 7.37P
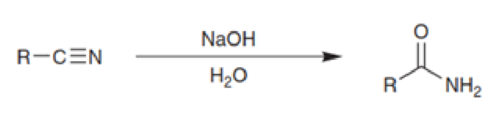
Based on everything we have just seen, propose a mechanism for the hydration of a nitrile under basic conditions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Propose a mechanism to account for the following finding:
Propose the mechanisms for each step of the synthesis below.
Provide a mechanism for the decarboxylation reaction below:
Chapter 7 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...
Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.5 - Prob. 7.23PCh. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - Prob. 7.33PCh. 7.5 - Prob. 7.34PCh. 7.5 - Prob. 7.35PCh. 7.5 - Prob. 7.36PCh. 7.6 - Based on everything we have just seen, propose a...Ch. 7.6 - Prob. 7.39PCh. 7.6 - Prob. 7.40PCh. 7.6 - Prob. 7.41PCh. 7.6 - Propose a mechanism for the following reaction:Ch. 7.6 - Prob. 7.44PCh. 7.6 - Prob. 7.45PCh. 7.6 - Prob. 7.46PCh. 7.7 - Prob. 7.48PCh. 7.7 - Prob. 7.49PCh. 7.7 - Prob. 7.50PCh. 7.7 - Prob. 7.51PCh. 7.7 - Prob. 7.52PCh. 7.7 - Prob. 7.53PCh. 7.7 - Prob. 7.55PCh. 7.7 - Prob. 7.56PCh. 7.7 - Prob. 7.57PCh. 7.7 - Prob. 7.58PCh. 7.7 - Prob. 7.59PCh. 7.7 - Prob. 7.60PCh. 7.7 - Prob. 7.61PCh. 7.7 - Prob. 7.62PCh. 7.7 - Prob. 7.63PCh. 7.7 - Prob. 7.64PCh. 7.7 - Prob. 7.65P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Complete and balance each equation. If no reaction occurs, write NO REACTION. a. KI(aq)+BaS(aq) b. K2SO4(aq)+Ba...
Introductory Chemistry (6th Edition)
a. Draw the resonance forms for SO2 (bonded OSO). b. Draw the resonance forms for ozone (bonded OOO). c. Sulfur...
Organic Chemistry (9th Edition)
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
CHEMISTRY-TEXT
In qualitative analysis, Ca2+ and Ba2+ are separated from Na+, K+, and Mg2+ by adding aqueous (NH4)2CO3 to a so...
General Chemistry: Atoms First
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a mechanism for the reaction belowarrow_forwardThe following compound undergoes Benzilic Acid Rearrangementto yield a hydroxyacid salt. Propose a mechanism for the reaction, write the major product, and provide an explanation as to the preference of migration of one R group over the other.arrow_forwardPropose mechanisms for the following base-catalyzed condensations, with dehydration.(a) 2,2-dimethylpropanal with acetaldehydearrow_forward
- Propose a reaction for the formation of the following products involving esterformation.arrow_forwardBisphenol A is made on a large scale by a condensation of phenol with acetone. Suggest an appropriate catalyst, and propose a mechanism for this reaction.arrow_forwardPropose mechanisms for the following base-catalyzed condensations, with dehydration. benzaldehyde with propionaldehydearrow_forward
- Propose the reaction mechanism for the two possible products formation from the following reaction of acetophenone and butanal using naoh.arrow_forwardpropose mechanisms for the following reactions.arrow_forwardPropose the starting reagents to obtain the following products: please don't provide hand written solution...arrow_forward
- Propose a mechanism for the acid-catalyzed hydration of propene. Remember that a mechanism must include curved arrows to show movement of electrons, as well as all intermediates.arrow_forward2,6-Dimethoxybenzoic acid was needed for a synthesis of the β-lactam antibiotic methicillin. Show how this carboxylic acid could be synthesized from 2-bromo-1,3-benzenediol.arrow_forwardPredict the product and propose a synthesis of p-bromoacetophenone from benzenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License