
Interpretation:
Whether the conversion of A to B is an exothermic reaction or endothermic reaction should be identified on increase in equilibrium concentration of B.
Concept Introduction:
A chemical equilibrium shifted towards forward direction or backward direction depends on the concentration of both reactant and products.
Exothermic reaction:
A
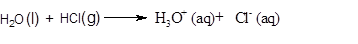
Endothermic reaction:
A chemical reaction in which energy is added when reactant converted into products known as endothermic reaction.
Temperature effect:
In a reaction, reactant molecule collides with each other and many bonds are break and formed in this process; all this process depend on the kinetic energy of molecules and as we increase the temperature the kinetic energy also increases as a result
Consider and equilibrium reaction:
Here, a, b, c and d are coefficient.
If we add excess concentration of c and d, then the chemical equilibrium will shifted in backward direction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Introduction to General, Organic and Biochemistry
- Problem 7-7 Consider the following equilibrium reaction for the decomposition of an aqueous solution of hydrogen peroxide: Oxygen has limited solubility in water (see the table in Chemical Connections 6A). What happens to the equilibrium after the solution becomes saturated with oxygen?arrow_forwardpls answer d, e, f thank u 2NO(g)+O2(g)⇌2NO2(g) + Heat a) where will the reaction be shifted (right or left) if there is an increase in temperature? b) increase in pressure c) increase in volume d.) addition of catalyst e.) cooling the system f.) What happens to the concentration of NO2 if there is a decrease in pressure? g.) removal of NO2 h.) adding more O2 i.) removal of NO?arrow_forwardFor the following reaction 3O₂(g) ⇌ 2O₃(g) Kc = 2.10 × 10⁻⁷ at a certain temperature. If [O₂] = 0.0365 M when at equilibrium, what is the equilibrium O₃ concentration? Answer ___ Marrow_forward
- The reactions below is carried out in a reaction vessel and allowed to reach equilibrium. Which reaction will favor reactant formation upon increasing the volume of the reaction vessel? A) N2 (g) + 3 H2 (g) ↔ 2NH3 (g) B) No right answer C) H2 (g) + F2 (g) ↔ 2 HF(g) D) CO(g) + NO2(g) ↔ CO2(g) + NO(g) E) C(s) + CO2(g) ↔ 2CO(g)arrow_forwardK=4.9x10-3 for the reaction CO2(g)+H2(g)⇄ CO(g)+H2O(g). If [CO2]=0.00025 M, [H2]=0.0010 M, & [CO]=0.000015 M, what is the concentration at equilibrium of H2O? Answer 8.2x10-5M I got 1.84x10^-14? Can someone explain this to me please.arrow_forwardThe equilibrium constant for the reaction 2 HF (g) ⇌ H₂ (g) + F₂ (g) is 0.360 at a particular temperature. What is the equilibrium constant for the equation ½ H₂ (g) + ½ F₂ (g) ⇌ HF (g)? ANswer _____arrow_forward
- For the reaction 2HI(g) <--> H2(g) + I2(g) Kc = 0.224 at 400K If 0.750 moles of HI are placed in a 5.00L flask and allowed to react , what is the [H2] when equilibrium is reached? (ICE problem. perfect square)arrow_forwardConsider the following reaction in which K = 8.3 x 10-2 at 250˚C.At equilibrium, C = 0.52 mol and [O2] = 0.75 M. What is the equilibrium concentration of CO?2C(s) + O2(g) <---> 2CO(g) Answer is 0.25 M, just want to see steps!arrow_forwardhello i need help with part b and part c only Consider the system: A(g) --> B(g) at 25°C. Assume that G°A = 8590 J/mol and G°B = 12846 J/mol. 1a) Calculate the value of the equilibrium constant for this reaction. the asnwer to part a is 1.80×10-1 1b) A non-equilibrium mixture of 1.00 mol of A(g) (partial pressure 1.00 atm) and 1.00 mol of B(g) (partial pressure 1.00 atm) is allowed to equilibrate at 25°C. Calculate the partial pressure of A(g) at equilibrium. (answer you Provided before which was 1.46atm is not correct) 1c) Calculate the partial pressure of B(g) at equilibrium. (answer you provided before which was 0.543atm is not correct)arrow_forward
- issue 6Choose the equilibrium in which the products are favored by a drop in pressure and the reactants favored by a drop in temperature.a) N2(g) + 3 H2(g) ⇔ 2 NH3(g) + 92.3 kJb) H2(g) + I2(g) + 51.8 kJ ⇔ 2 HI(g)c) PCl3(g) + Cl2(g) ⇔ PCl5(g) ΔH = -84.2 kJd) 2 NO2(g) ⇔ 2 NO(g) + O2(g) ΔH = +54 kJarrow_forwardConsider the reaction below, for which K = 4.2 × 10–31 at 27 °C. N2 (g) + O2 (g) ⇌ 2NO (g) What is the value of K for the equilibrium below?4NO (g) ⇌ 2N2 (g) + 2O2 (g) Select one: a. 8.4 × 1031 b. 2.4 × 1030 c. 1.8 × 10–61 d. 8.4 × 10–31 e. 5.7 × 1060arrow_forwardIn which circumstances, will the value of the equilibrium constant Kp decrease as temperature increases for a reaction in gas phase? under no circumstances. if ΔH > 0. if more moles of gas are on product side of reaction. if more moles of gas are on reactant side of reaction. (Your answer) if ΔH < 0. (Correct answer)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


