
a)
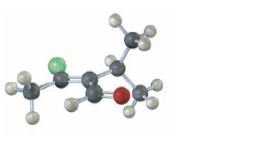
Interpretation:
The skeletal structure of the compound given in the model is to be drawn and the stereochemistry of the double bond to be assigned as E or Z.
Concept introduction:
The isomer that has the higher ranked groups on each carbon on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on the opposite sides, the
In skeletal structures the carbon and hydrogen atoms are not usually shown. Instead carbon atoms are assumed to be at each intersection of two lines and at the end of each line. Hydrogen atoms required are fitted mentally having in mind the valence of carbon is four. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of the compound given in the model and to assign the stereochemistry of the double bond as E or Z.
b)
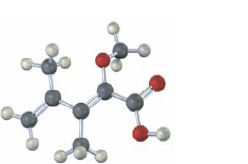
Interpretation:
The skeletal structure of the compound given in the model is to be drawn and the stereochemistry of the double bond to be assigned as E or Z.
Concept introduction:
The isomer that has the higher ranked groups on each carbon on the same side of the double bond is said to have Z configuration. If the higher ranked groups are on the opposite sides, the alkene is said to have E configuration.
In skeletal structures the carbon and hydrogen atoms are not usually shown. Instead carbon atoms are assumed to be at each intersection of two lines and at the end of each line. Hydrogen atoms required are fitted mentally having in mind the valence of carbon is four. Atoms other than carbon and hydrogen are shown.
To draw:
The skeletal structure of the compound given in the model and to assign the stereochemistry of the double bond as E or Z.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry
- 3H. Put it all together. Predict the E1 products of the following reaction. Label alkenes as E/Z. 4. Predict the E1 products of the following reaction. Label akenes as E/Z.arrow_forwardWhat stereoisomers are obtained from the reaction of each of the following alkenes with OsO4 followed by aqueous H2O2? a. trans-2-butene b. cis-2-butene c. cis-2-pentene d. trans-2-pentenearrow_forwardName (including E/Z stereochemistry) the five alkenes that can produce 3-bromo-3-methylhexane on reaction with HBr. Draw the skeletal structure of each molecule.arrow_forward
- What are the major products from the reaction 2-phenyl propene and bromine in water? Show the stereochemistry of the products and assign the configuration R or Sarrow_forwardThe bicyclic alkene P can be prepared by thermal electrocyclic ring closure from cyclodecadiene Q or by photochemical electrocyclic ring closure from cyclodecadiene R. Draw the structures of Q and R, and indicate the stereochemistry of the process by which each reaction occurs.arrow_forwardAccount for the regioselectivity and stereoselectivity observed when 1-methylcyclopentene is treated with reagent Q.Hg(OAc)2 in H2Oarrow_forward
- Consider a reaction where cis-but-2-ene is treated with a peroxy acid followed by OH- /H20. Draw the structure of one product that is formed in the reaction, including correct stereochemistry.arrow_forwardName the alkene below.Use ONLY E/Z designators to indicate stereochemistry.H2C=C=CHCH3arrow_forwardWhat organic product is formed when 1‑methylcyclopentene is treated with NMMO in the presence of H2O and a catalytic amound of OsO4? Clearly show stereochemistry by drawing a wedge and dashed bond for each chiral carbon. Only draw one stereoisomer if more than one can be formed.arrow_forward
- Ignoring stereochemistry, draw the alkylborane formed from the addition of one equivalent of BH3 to the alkene. The alkylborane formed in Part 1 is further treated with H2O2 and HO−. Draw the two stereoisomers of the final product of the reaction. Include stereochemistry where relevant. How many stereocenters are formed from the reaction? What is the relationship between the stereoisomers?arrow_forwardWhat is the stereochemistry of the product made by the reaction of but-2-yne with 1 equivalence of H-Br? CH3 - C ≡ C - CH3arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

