
a)
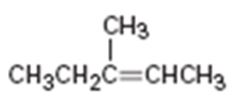
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to 3-methyl-3-butene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to 3-methyl-3-butene assuming that Markovnikov’s rule is valid.
b)
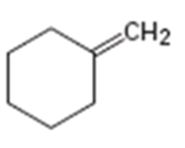
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to methylidenecyclohexene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an alkene, the H attaches itself with the carbon with fewer alkyl substituents and the X attaches itself with the carbon with more alkyl substituents.
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to methylidenecyclohexene assuming that Markovnikov’s rule is valid.
c)
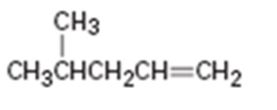
Interpretation:
Assuming Markovnikov’s rule is valid, the major alcohol product obtainable by the acid-catalyzed addition of water to 4- methyl-1-pentene is to be predicted.
Concept introduction:
Markovnikov’s rule can be used to decide on the orientation in electrophilic addition reactions. According to this rule, in the addition of HX to an alkene, the H attaches itself with the carbon with fewer alkyl substituents and the X attaches itself with the carbon with more alkyl substituents.
To state:
The major alcohol product obtainable by the acid-catalyzed addition of water to 4- methyl-1-pentene assuming that Markovnikov’s rule is valid.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry
- Predict the products of reaction, if any, of isopropyl alcohol with each of the following reagents:arrow_forwardWhat two alkenes give rise to each alcohol as the major product of acid-catalyzed hydration?arrow_forwardComplete the following transformations by indicating the reactant structure, all necessary reagents, or the major organic product(s).arrow_forward
- Which alkene would the following alcohols form upon dehydration reaction using POCl3 inpyridine? Indicate the major productarrow_forwardPropane (CH3CH2CH3) reacts with bromine (Br2) in the presence of ultra-violet (UV) light. Draw the displayed formulae of one of the monobrominated organic products formed in this reaction.arrow_forwardPlease help in answering the following questions ~Which of the following is the basis of the classification of alcohols?A. Based on the number of hydroxyl groups attached.B. Based on the number of carbon atoms which are directly attached to the carbon thatis bonded with the -OH group.C. BothD. Neither ~Which of the following carboxylic acids will yield the least amount of protons in an aqueoussolution?A. 2-chlorobutanoic acidB. Propanoic acidC. Butanoic acidD. 2-methylbutanoic acidarrow_forward
- Assign R/S to the following organic compounds then give its IUPAC Nomenclaturearrow_forwardAlcohols, ethers and phenols Give each of the following compounds an appropriate name. d. CH3CH2OCH2(CH3)C3H7 (n)e. CH3OC6H9 (neo)arrow_forwardWhat will be the resulting products structure when 1-butanol is oxidized using pyridinium chlorochromate (PCC)?arrow_forward
- Directions: Identify what type of organic reaction is being represented by each item.Choose from the basic types of organic reactions (e.g. combustion, addition,condensation and saponification reaction)._____________1. Hydrocarbons reacts with oxygen gas producing water andcarbon dioxide as products._____________2. Reaction of water to an alkene leading to the removal of thedouble bond and production of an alcohol._____________3. A carboxylic acid and an alcohol react forming a new bondwith the simultaneous loss of water molecule, hence the formationof a new compound._____________4. The production of soap from fats_____________5. The production of esters from alcohol and carboxylic acidarrow_forwardplease help with the following question: Draw a structural formula for the major organic product AND intermediate of the following reaction:arrow_forwardIdentify which of the statements is/are correct. (i) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (j) The molecular formula of the smallest carboxylic acid is C2H4O2.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
