
Interpretation:
The purposeof the use of concentrated
Concept Introduction:
The preparation of aspirin takes place by the reaction of salicylic acid and acetic anhydride represented as follows:
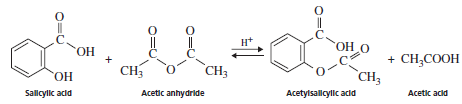
The -OH group of the salicylic acid reacts with acetic anhydride to form ester thus, the formation of aspirin is an esterification reaction.
Answer to Problem 1Q
To activate the reaction.
Explanation of Solution
The acid serves to protonate one double bonded oxygen on the acetic anhydride this makes the reaction with the salicylic acid faster.
The mechanism is represented as follows:
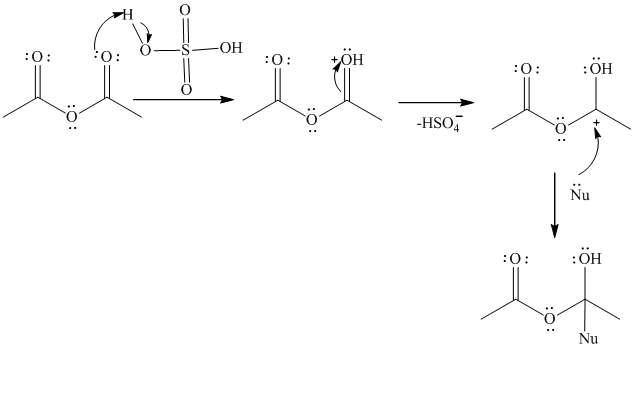
Thus, the acid acts as the catalyst during the esterification reaction by making the carbonyl group of anhydrous acetic acid more reactive.
Thus, the purpose of the use of concentrated
Want to see more full solutions like this?
Chapter 8 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- What is the role of Sodium metal in Lassaigne's extraction?arrow_forwardExplain what happened to the salt (the inorganic impurity) during the extraction process. Where does it go and why?arrow_forward4.The process of removing SO2 by injecting powedered limestone which is converted to calcium oxide?arrow_forward
- The reaction for the magnesium cation with 8-hydroxyquinoline is carried out in the presence of: A) Nitric acid B) Sodium hydroxide C) A solution of ammonia and ammonium chloride D) Sulfuric acid E) Sodium acetatearrow_forward1. What is the element indicated by the residue formed in the charring test? 2. How do the conductivity reading relate to the nature of the chemical bonds present in table sugar and Salt water? Justify your answer. 3. What is the role of Sodium metal in Lassaigne's extraction?arrow_forwardWhat is the function of resazurin in the reduction test and what type of chemical reactions is involved?arrow_forward
- What is the best choice of solvent for the formation of phenylmagnesium bromide by the reaction of bromobenzene with magnesium? Explain whyarrow_forwardwhich will produce an oxidation product with potassium permanganate that is insoluble in sodium hydroxide?arrow_forwardWhat would the KMnO4 reaction be?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

