
Concept explainers
How many
How many yield 2,3-dimethylbutane?
How many yield methylcyclobutane?
Interpretation:
The number of alkenes which are required to produce the given alkanes on catalytic hydrogenation is to be determined.
Concept introduction:
On catalytic hydrogenation, alkenes get converted to the corresponding alkanes with the same number of carbon atoms.
In hydrogenation reaction, one hydrogen atom gets attached to each of the double bonded carbon atoms.
To accelerate the rate of hydrogenation, the metal catalyst provides an alternative pathway involving low activation energy steps.
The alkane that is formed contains two hydrogen atoms more than the corresponding alkene and these two hydrogen atoms are added on the adjacent carbon atoms in the alkene.
Answer to Problem 25P
Solution:
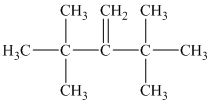
a) Only one alkene can produce
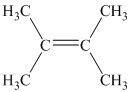
b) Two alkenes can produce
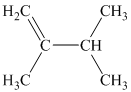
c) Three alkenes can produce methylcyclobutane upon catalytic hydrogenation.
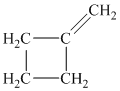
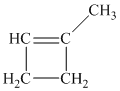
Explanation of Solution
a) The structure of
Hydrogenation of alkenes adds one hydrogen atom on each carbon atom of the double bonded carbon atoms. So in the alkane, these two carbon atoms (previously double bonded) should be attached to at least one hydrogen atom each.
In
The alkane structure is symmetric, and carbon atoms
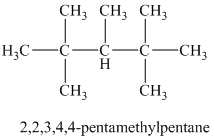
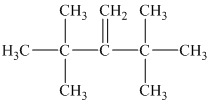
b) The structure of
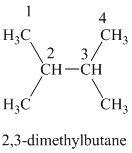
The hydrogen of alkenes adds one hydrogen atom on each carbon atom of the double bonded carbon atoms. So in the alkane, these two carbon atoms (previously double bonded) should be attached to at least one hydrogen atom each.
In
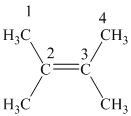
The
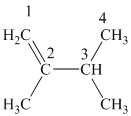
The alkane structure is symmetric, and the carbon atoms
Hence, two alkenes can produce
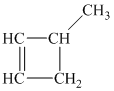
c) The structure of methylcyclobutane is shown below:
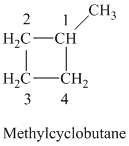
The hydrogen of alkenes adds one hydrogen atom on each carbon atom of the double bonded carbon atoms. So in the alkane, these two carbon atoms (previously double bonded) should be attached to one at least one hydrogen atom each.
In methylcyclobutane, the
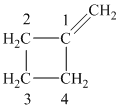
The
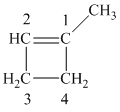
The
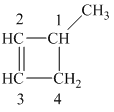
The alkane structure is symmetric. This means, there cannot be any other distinct alkene structure which would produce methylcyclobutane upon catalytic hydrogenation.
Hence, three alkenes can produce methylcyclobutane upon catalytic hydrogenation.
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY,VOL.1 >CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





