
Concept explainers
Interpretation:
The wrong point in the given Lewis structures is to be stated, and the correct Lewis structure of the molecule is to be drawn.
Concept Introduction:
Lewis structure is a representation of the bonding and non-bonding electron pairs present in the outermost shell of all atoms present in the molecule.
The number of bonds formed by an atom in the molecule is determined by the valence electrons pairs.
Answer to Problem 54QP
Solution:
a)
The double bond is present between the carbon and nitrogen atoms.
The correct Lewis structure is as follows:
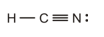
b)
The double bond is present between the carbon and hydrogen atoms.
The correct Lewis structure is as follows:
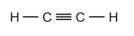
c)
A single bond is present between the
The correct Lewis structure is as follows:
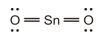
d)
The lone pair of electrons on boron.
The correct Lewis structure is as follows:
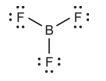
e)
The double bond between the oxygen and fluorine atoms.
The correct Lewis structure is as follows:
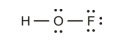
f)
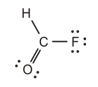
The single bond between the carbon and oxygen atoms.
The correct Lewis structure is as follows:
g)
The lone pair of electrons of the nitrogen atom is missing.
The correct Lewis structure is as follows:
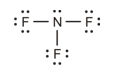
Explanation of Solution
a)
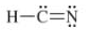
The given Lewis structure is as follows:
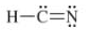
The electronic configurations of hydrogen, carbon, and nitrogen in
Carbon has four electrons in its outermost shell and requires four electrons to complete its outermost shell of electrons, while nitrogen requires three electrons to complete its octet and hydrogen requires one electron to obtain its fully-filled electronic configuration. Therefore, the Lewis structure of
The correct Lewis structure of
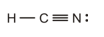
Hence, the given Lewis structure of
b)
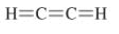
The given Lewis structure is as follows:
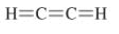
The electronic configurations of carbon and hydrogen in
The carbon atom contains four electrons in its outermost shell and the hydrogen atom contains one valence electron in its
The correct Lewis structure of
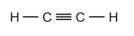
Hence, the given Lewis structure of
c)
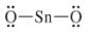
The given Lewis structure is as follows:
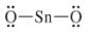
The electronic configurations of tin and oxygen in
A carbon atom has a tendency to form four bonds because of the presence of four valence electrons in its outermost shell, while oxygen has a tendency to form two bonds due to the presence of two electrons in its outermost shell. The Lewis structure of
The correct Lewis structure of
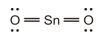
Hence, the given Lewis structure of
d)
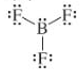
The given Lewis structure is as follows:

The electronic configurations of boron and fluorine in
The boron atom contains three electrons in its outermost shell and the fluorine atom contains five electrons in its
The Lewis structure of

Hence, the given Lewis structure of
e)
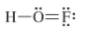
The given Lewis structure is as follows:
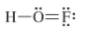
The electronic configurations of hydrogen, oxygen, and fluorine in
Hydrogen has a tendency to form one bond because of the presence of one electron in its outermost shell, fluorine has a tendency to form one bond because of the presence of five electrons in its
The correct Lewis structure of
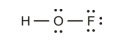
Hence, the given Lewis structure of
f)
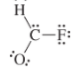
The given Lewis structure is as follows:

The electronic configurations of fluorine, carbon, oxygen, and hydrogen in
The fluorine atom has a tendency to form one bond because of the presence of five valence electrons in its

Hence, the given Lewis structure of
g)
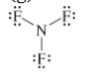
The given Lewis structure is,

The electronic configurations of nitrogen and chlorine in
The nitrogen atom contains three valence electrons in its
The correct Lewis structure of
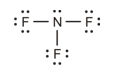
Hence, the given Lewis structure of
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry
- For three simple molecules of your own choice, apply the rules for writing Lewis structures. Write your discussion as if you are explaining the method to someone who is not familiar with Lewis structures.arrow_forwardA compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.arrow_forwardMethylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is In this molecule, which is the (a) weakest carbon-containing bond? (b) strongest carbon-containing bond? (c) most polar bond?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





