
(a)
Interpretation:
The two expressions for Doppler broadening and Doppler half-width needs to be shown equivalent to each other.
Concept introduction:
The equation for the half-width for Doppler broadening Δλ0 of an atomic line can be used to study line broadening in a low − pressure laser-induced plasma.
Explanation of Solution
The change in wavelength at the center of the emission line can be represented as follows:
Here,
Similarly, the Doppler half-width can be calculated as follows:
Here,
Also,
(b)
Interpretation:
The half-width for Doppler broadening needs to be determined for 4s to 4p transition for nickel atom.
Concept introduction:
Doppler bordering is happened due to the Doppler effect caused by a distribution of velocities of atomic molecules.
Answer to Problem 8.12QAP
The half-width = 7934 nm and
Explanation of Solution
Given information:
Calculation:
The Doppler half-width can be calculated as follows:
(c)
Interpretation:
The natural line width for the above transition needs to be determined, assuming that the lifetime of the excited state is
Concept introduction:
Natural line width is associated with the decay time (Natural life-time) and it is a minimum line width that does not contain effects such as collisional and Doppler broadening.
Answer to Problem 8.12QAP
Natural line width =
Explanation of Solution
Natural line width can be calculated as follows:
Putting the values,
(d)
Interpretation:
To show that the relativistic expression is consistent with the mentioned equation given for the low atomic speeds.
Concept introduction:
When compared with the speed of light, atomic speed is very low.
Explanation of Solution
When the atomic speed very low V is considerably small when compared to the c, that of the speed of light. Hence the above mentioned equation could be written as shown below. Hence, at low velocities, relativistic kinetic energy reduces to classical kinetic energy. No object with mass can achieve the speed of light because an infinite amount of energy input and an infinite amount of work is required to accelerate a mass to the speed of light.
(e)
Interpretation:
The speed of an iron atom the 4s to 4p transition at 385.9911 nm should be determined.
Concept introduction:
The rest wavelength of Nickel is 410 nm. The formula used is:
Answer to Problem 8.12QAP
Explanation of Solution
Given information:
Calculation:
(f)
Interpretation:
The fraction of a sample of iron atoms at 10,000 K that would have the velocity calculated in part e should be computed.
Concept introduction:
Natural line width is associated with the decay time. It is a minimum line width that does not contain effects such as collisional and Doppler broadening.
Answer to Problem 8.12QAP
Explanation of Solution
Given information:
Calculation:
(g)
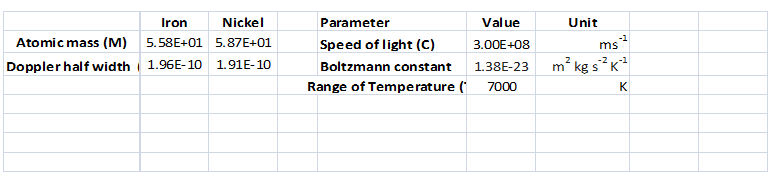
Interpretation:
A spreadsheet should be created to calculate the Doppler half-width
Concept introduction:
Doppler bordering is happened due to the Doppler effect caused by a distribution of velocities of atomic molecules.
Answer to Problem 8.12QAP
Refer the spreadsheet
Explanation of Solution
Given information:
Calculation:
(h)
Interpretation:
The four sources of pressure broadening should be listed by consulting the paper by Gornushkin et al. (note 10).
Explanation of Solution
The interaction of the surrounding particles with the radiating atom is the major source of pressure line broadening, which causes a phase shift and a frequency disturbance.
The most important cases of interaction are:
- linear Starkeffect, p = 2;
- resonance interaction between identical particles, p = 3;
- quadratic Stark effect, p = 4,
- van der Waals interaction, p = 6.
The superposition problems are avoided by two approximations:
- ‘nearest neighbor approximation’, in this the considered interaction is interaction with the closest perturber.
- The impact or collision concept, in which moving perturbers act sequentially in time.
Want to see more full solutions like this?
Chapter 8 Solutions
Principles of Instrumental Analysis
- Why is 133C-133C spin-spin splitting not observed in ordinary organic compounds?arrow_forwardThe molar absorption coefficient of a solute at 540 nm is 386 dm3 mol−1 cm−1. When light of that wavelength passes through a 5.00 mm cell containing a solution of the solute, 38.5 per cent of the light was absorbed. What is the molar concentration of the solute?arrow_forwardCalculate the ratio A/B for transitions with the following characteristics: (i) 500 MHz radiofrequency radiation, (ii) 3.0 cm microwave radiation.arrow_forward
- quantum chemistry Estimate the transmission coefficient for an electron tunneling through a barrier of height 2 eV and width 20 pm when its energy is 1.2 eVarrow_forwardCalculate the wavlength (in nm) of electromagnetic radiation with a frequency of 5.8 x 1014 s-1.arrow_forwardAssume that the states of the π electrons of a conjugated molecule can be approximated by the wavefunctions of a particle in a one-dimensional box, and that the magnitude of the dipole moment can be related to the displacement along this length by μ = −ex. Show that the transition probability for the transition n = 1 → n = 2 is non-zero, whereas that for n = 1 → n = 3 is zero. Hints: The following relation will be useful: sin x sin y = 1/2cos(x − y) − 1/2cos(x + y). Relevant integrals are given in the Resource section.arrow_forward
- 3 Calculate the frequencies and wavelengths for the rotational transition J = 0 → J = 1 and J = 4 → J = 5 for the HCl molecule. The internuclear distance is Re = 0.12745 nm. What is the frequency shift between the two isotopomers H35Cl and H37Cl for the two transitions?arrow_forwardWhat is the wavenumber of the radiation emitted when a hydrogen atom makes a transition corresponding to a change in energy of 10.20 eV?arrow_forwardA 2.0 mm cell was filled with a solution of benzene in a non-absorbing solvent. The concentration of the benzene was 0.010 mol dm−3 and the wavelength of the radiation was 256 nm (where there is a maximum in the absorption). Calculate the molar absorption coefficient of benzene at this wavelength given that the transmission was 48 per cent. What will the transmittance be through a 4.0 mm cell at the same wavelength?arrow_forward
- Which of the three vibrations of an AB2 molecule are infrared or Raman active when it is (i) angular, (ii) linear?arrow_forwardCalculate the wavenumber of the first overtone transition w ith v = 2 ~ 0 in nitric oxide. NO. given that the valueof the vibrational wavenumber is v = 1904.03 em·' and theanharmonicity constant is x, = 0.0073.arrow_forward(a) Express the moment of inertia of an octahedral AB6 molecule in terms of its bond lengths and the masses of the B atoms. (b) Calculate the rotational constant of 32S19F6 , for which the S—F bond length is 158 pm.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

