
Concept explainers
For the reaction
(a) Predict the enthalpy of reaction from the average bond enthalpies in Table 8.6. (b) Calculate the enthalpy of reaction from the standard enthalpies of formation (see Appendix 2) of the reactant and product molecules, and compare the result with your answer for part (a).
Interpretation:
The
Concept Introduction:
The enthalpy of the reaction by using the bond enthalpy is calculated by the expression, which is as:
Here,
The enthalpy of the reaction by using the standard enthalpy of formation is calculated by the expression, which is as:
Here,
Rules to write the Lewis structure are as:
The skeletal structure of the compound is drawn in which the less electronegative element is placed as the central atom, which is surrounded by substituent atoms.
The total number of valence electrons for the compound is determined.
Subtract two electrons for each bond in the skeletal structure from the total number of valence electrons to know the number of remaining electrons.
Complete the octet of each terminal atom by placing a pair of electrons from the remaining electrons.
If any of the electrons are remaining after completing the octet of terminal atoms, place the remaining electrons as a pair on the central atom.
Answer to Problem 83QP
Solution:
The
The
Explanation of Solution
a)The enthalpy of reaction from the average bond enthalpies.
The given reaction is
The bond enthalpy value of
The bond enthalpy value of
The bond enthalpy value of
The bond enthalpy value of
The bond enthalpy value of
The Lewis structure of the given reaction is drawn in order to determine which bond is formed and which is broken.
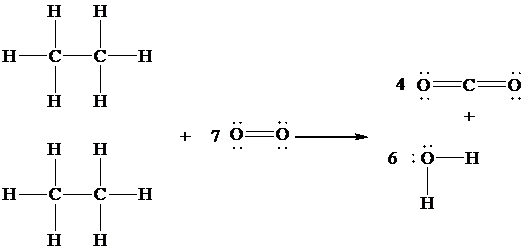
In the given reaction,
The
Substitute the value
b) The enthalpy of reaction from the standard enthalpies of formation
The given reaction is
The value of
The value of
The value of
The value of
Here,
The value of change in the enthalpy for atoms in their standard state is zero. In the reaction,
Substitute
The values of
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry
- The reaction of quicklime, CaO, with water produces slaked lime, Ca(OH)2, which is widely used in the construction industry to make mortar and plaster. The reaction of quicklime and water is highly exothermic: CaO(s)+H2O(l)Ca(OH)2(s)H=350kJmol1 (a) What is the enthalpy of reaction per gram of quicklime that reacts?. (b) How much heat, in kilojoules, is associated with the production of 1 ton of slaked lime?arrow_forwardWith a platinum catalyst, ammonia will burn in oxygen to give nitric oxide, NO. 4NH3(g)+5O2(g)4NO(g)+6H2O(g);H=906kJ What is the enthalpy change for the following reaction? NO(g)+32H2O(g)NH3(g)+34O2(g)arrow_forwardIs the following reaction the appropriate one to use in determining the enthalpy of formation of methane, CH4(g)? Why or why not? C(g)+4H(g)CH4(g)arrow_forward
- Which of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation?arrow_forwardGiven the following data calculate H for the reaction On the basis of the enthalpy change, is this a useful reaction for the synthesis of ammonia?arrow_forwardThe thermochemical equation for the burning of methane, the main component of natural gas, is CH4(g)+2O2(g)CO2(g)+2H2O(l)H=890kJ (a) Is this reaction endothermic or exothermic? (b) What quantities of reactants and products are assumed if H = 890 kJ? (c) What is the enthalpy change when 1.00 g methane burns in an excess of oxygen?arrow_forward
- The enthalpy change for the reaction of hydrogen gas with fluorine gas (o produce hydrogen fluoride is 542 U for the equation as written: mg src=Images/HTML_99425-10-41QAP_image001.jpg alt="" align="top"/> l type='a'> What is the enthalpy change per mole of hydrogen fluoride produced? Is the reaction exothermic or endothermic as written? What would be the enthalpy change for the reverse of the given equation (that 1%, for the decomposition of HF into its constituent elements)?arrow_forward9.99 The chemical reaction BBr3(g)+BCl3(g)BBr2Cl(g)+BCl2Br(g) , has an enthalpy change very close to zero. Using Lewis structures of the molecules, all of which have a central boron atom, provide a molecular-level description of why H for this reaction might be very small.arrow_forward9.41 Under what conditions does the enthalpy change equal the heat of a process?arrow_forward
- For the reactions of molecular hydrogen with fluorine and with chlorine: (a) Calculate the enthalpy change for breaking all the bonds in the reactants. (b) Calculate the enthalpy change for forming all the bonds in the products. (c) From the results in parts (a) and (b), calculate the enthalpy change for the reaction. (d) Which reaction is most exothermic?arrow_forwardA commercial process for preparing ethanol (ethyl alcohol), C2H5OH, consists of passing ethylene gas. C2H4, and steam over an acid catalyst (to speed up the reaction). The gas-phase reaction is Use bond enthalpies (Table 9.5) to estimate the enthalpy change for this reaction when 37.0 g of ethyl alcohol is produced.arrow_forwardThe standard enthalpies of formation for S(g), F(g), SF4(g), and SF6(g) are +278.8, +79.0, 775, and +1209 KJ/mol, respectively. a. Use these data to estimate the energy of an SF bond. b. Compare your calculated value to the value given in Table 3-3. What conclusions can you draw? c. Why are the Hf values for S(g) and F(g) not equal to zero, since sulfur and fluorine are elements?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





