
(a)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which
(a)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 1.
The major product of the given reaction is shown in Figure 2.
Explanation of Solution
When
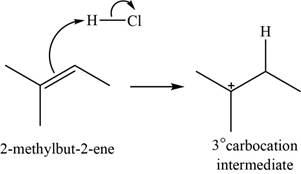
Figure 1
The chloride ion acts as a nucleophile that attacks on the electrophilic tertiary carbocation and results in the formation of
The major product of the given reaction is,
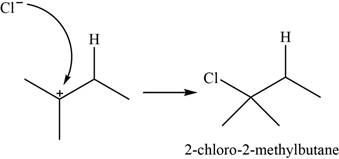
Figure 2
(b)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(b)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 3.
The major product of the given reaction is shown in Figure 4.
Explanation of Solution
When
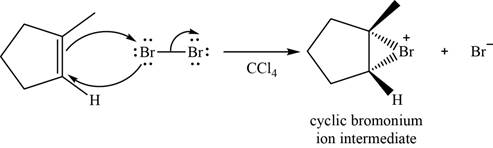
Figure 3
Bromide ion opens the bromonium ion intermediate at the more substituted carbon atom and forms
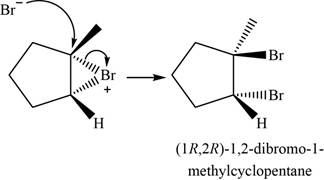
Figure 4
(c)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(c)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 5.
The major product of the given reaction is shown in Figure 6.
Explanation of Solution
When
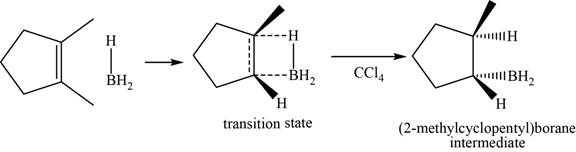
Figure 5
When
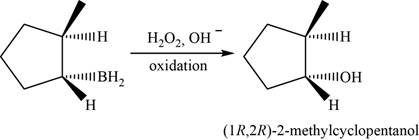
Figure 6
(d)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(d)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 7.
The major product of the given reaction is shown in Figure 8.
Explanation of Solution
When
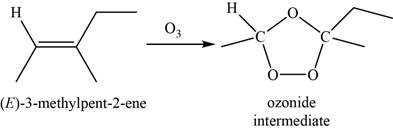
Figure 7
Now, the ozonide intermediate reacts with dimethyl sulfide and forms acetaldehyde and
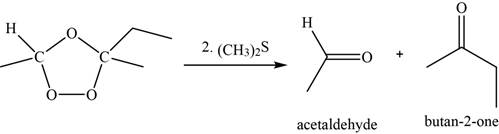
Figure 8
(e)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(e)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 9.
Explanation of Solution
When alkene reacts with
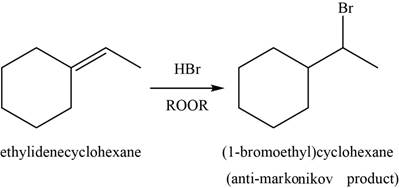
Figure 9
(f)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(f)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 10.
Explanation of Solution
When alkene reacts with
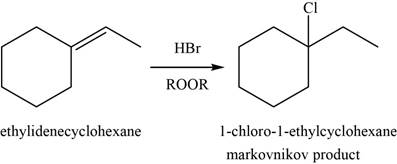
Figure 10
(g)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(g)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 11.
Explanation of Solution
When an alkene reacts with peroxyacid, it forms an
The major product of the given reaction is,
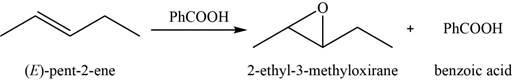
Figure 11
(h)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(h)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 12.
The major product of the given reaction is shown in Figure 13.
Explanation of Solution
Alkene undergoes
The structure of an intermediate is given as,
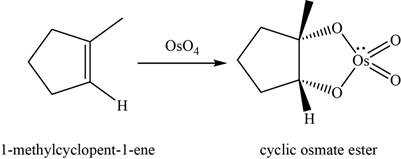
Figure 12
Now, the cyclic osmate undergoes oxidation in the presence of
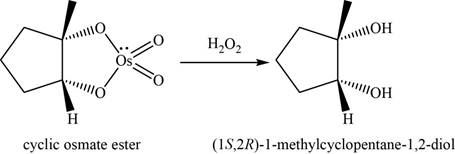
Figure 13
(i)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(i)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 14.
Explanation of Solution
The major product of the given reaction is,
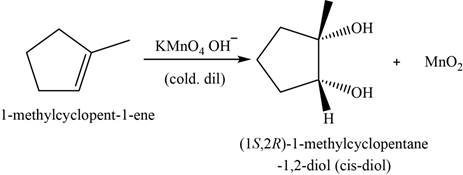
Figure 14
(j)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(j)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 15.
The major product of the given reaction is shown in Figure 16.
Explanation of Solution
When an alkene reacts with peroxyacid, it forms an epoxide or oxirane. Here,
The structure of an intermediate is given as,
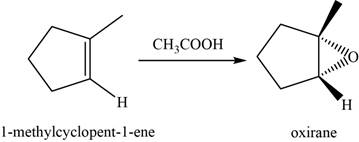
Figure 15
The oxirane is opened at more substituted carbon atom in the presence of an acid
The major product of the given reaction is,
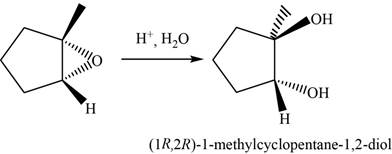
Figure 16
(k)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(k)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 17.
Explanation of Solution
Alkene undergoes oxidative cleavage in the presence of hot concentrated
The major product of the given reaction is,
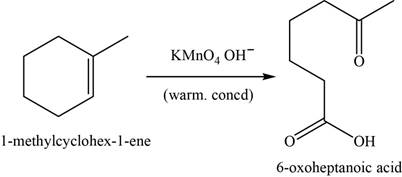
Figure 17
(l)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(l)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 18.
Explanation of Solution
Alkene undergoes ozonolysis to form carbonyl compounds. Here,
The major product of the given reaction is,
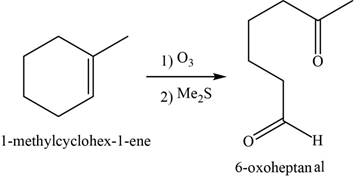
Figure 18
(m)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(m)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 19.
Explanation of Solution
In this reaction,
The major product of the given reaction is,
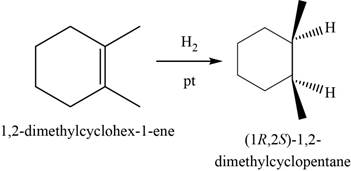
Figure 19
(n)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(n)
Answer to Problem 8.46SP
The structure of an intermediate and the major product of the given reaction are shown in Figure 20.
Explanation of Solution
This reaction is an acid catalyzed hydration of alkene and forms
The structure of an intermediate and the major product of the given reaction is,
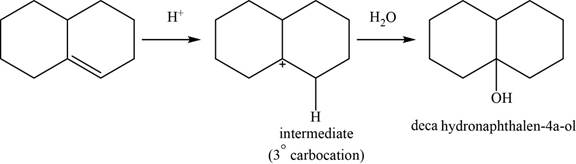
Figure 20
(o)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(o)
Answer to Problem 8.46SP
The major product of the given reaction is shown in Figure 21.
Explanation of Solution
The given reaction is an olefin metathesis. The major product of the given reaction is,
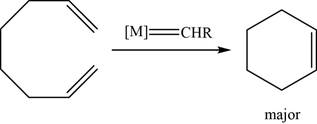
Figure 21
(p)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(p)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 22.
The major product of the given reaction is shown in Figure 24.
Explanation of Solution
Alkene reacts with mercuric acetate and forms mercurinium ion intermediate.
The structure of an intermediate is given as,
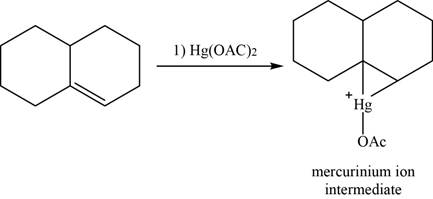
Figure 22
Now, water molecule acts as a nucleophile that opens the mercurinium ion at the most substituted carbon atom.
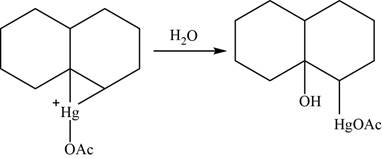
Figure 23
Now, organomercurial alcohol undergoes demercuration in the presence of
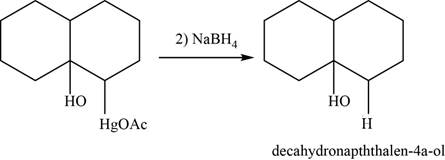
Figure 24
(q)
Interpretation:
The major product of the given reaction and the structure of any intermediate are to be predicted.
Concept introduction:
Substitution reactions are the reactions in which there is a replacement of an atom or a functional group by another atom or a functional group.
Elimination reactions are the reaction in which alkenes are prepared. In this two substituents are eliminated by either one step or two step mechanism.
(q)
Answer to Problem 8.46SP
The structure of an intermediate is shown in Figure 25.
The major product of the given reaction is shown in Figure 26.
Explanation of Solution
Alkene reacts with chlorine and forms chloronium ion intermediate.
The structure of an intermediate is given as,
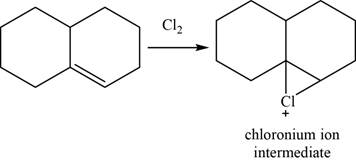
Figure 25
Now, the water molecule acts as a nucleophile that opens the chloronium ion intermediate at more substituted carbon atom. The major product of the given reaction is,
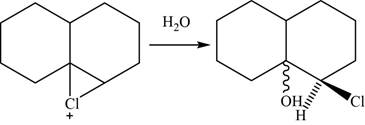
Figure 26
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
- Predict the products and show the mechanisms for the following reactions. Please indicate the correct stereochemistry where necessary.arrow_forwardComplete each of the following reactions by providing missing reagents, starting materials, intermediates or major products, where applicable. Clearly indicate the stereochemistry if necessary.arrow_forwardGive the structure of the product and/or intermediates of the following reactions. Indicate, where appropriate, both regiochemistry and stereochemistry.arrow_forward
- Write the mechanisms of the following reactions and examine the products as stereoisomeric.arrow_forwardPredict the products and include stereochemistry.arrow_forwardPredict the major organic product(s) of the following reactions or provide the reagents needed to complete the following transformations.arrow_forward
- Suggest a detailed mechanism for the reaction below. Represent the product in the most stable conformation and also its stereochemistry.arrow_forwardEstablish the reagents necessary to carry out the following synthesis, also propose a reasonable reaction mechanism for the formation of the product.arrow_forwardPlease provide mechanism and describe stereochemistry of productsarrow_forward
