
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.7, Problem AQ
Linoleic acid is shown below. What makes this fatty acid particularly susceptible to autoxidation?
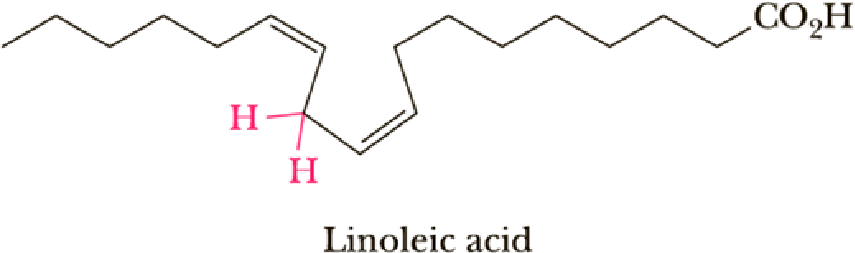
- 1. The red C—H bond has a low
bond dissociation energy because it is doubly allylic. - 2. The red C—H bond has a high bond dissociation energy because it is doubly allylic.
- 3. The red C—H bond is the most accessible to reaction with O2 because it is the least sterically crowded C—H bond.
- 4. Both 2 and 3.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Illustrate the dehydration of CH3CH2OH to CH2=CH2—An endothermic reaction ?
1. Bromine test is a test used to determine the degree of unsaturation in fatty acids. A colorless solution indicates a positive result. Which of the following fatty acids will not give a colorless solution?
oleic acid
Linoleic acid
Lauric acid
Linolenic acid
2. The following can cause the breaking of hydrogen bond, except?
addition of silver nitrate
heating
agitation
addition of acetic acid
none of the above
1. Some marine plankton contain triacylglycerols formed from the polyunsaturated fatty acid (PUFA) such as: CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2COOH
a) Draw the skeletal structure of the given PUFA showing the preffered stereogenic arrangement for a naturally-occuring lipid at each double bond.
b) Write the systematic name of the fatty acid.
c) What type of omega-n acid is this fatty acid?
d) Would you expect this fatty acid to be a soild or liquid at room temperature?
Chapter 8 Solutions
Organic Chemistry
Ch. 8.2 - Prob. 8.1PCh. 8.4 - Name and draw structural formulas for all...Ch. 8.4 - Using the table of bond dissociation enthalpies in...Ch. 8.5 - Prob. 8.4PCh. 8.6 - Given the solution to Example 8.5, predict the...Ch. 8.7 - Prob. 8.6PCh. 8.7 - Linoleic acid is shown below. What makes this...Ch. 8.7 - Prob. BQCh. 8.7 - Prob. CQCh. 8.7 - The strength of the HO bond in vitamin E is weaker...
Ch. 8.7 - Prob. EQCh. 8.8 - Prob. 8.7PCh. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10PCh. 8 - Prob. 8.11PCh. 8 - Account for the fact that among the chlorinated...Ch. 8 - Name and draw structural formulas for all possible...Ch. 8 - Prob. 8.14PCh. 8 - There are three constitutional isomers with the...Ch. 8 - Following is a balanced equation for bromination...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Cyclobutane reacts with bromine to give...Ch. 8 - Prob. 8.21PCh. 8 - Following is a balanced equation for the allylic...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - The major product formed when methylenecyclohexane...Ch. 8 - Prob. 8.26PCh. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Write the products of the following sequences of...Ch. 8 - Using your reaction roadmap as a guide, show...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - Prob. 8.34P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the following fatty acid, and tell whether it is more likely to be found in peanut oil or in red meat:arrow_forwardHow would you expect the melting point of eicosapentaenoic acid[CH3CH2(CH=CHCH2)5(CH2)2COOH] to compare with the melting points of the fatty acids listed in attached filearrow_forwardCalculate the theoretical yield of 2mL of vegetable oil saponification reaction with 2mL of 6M NaOH and 40mL of saturated salt solution(NaCl). GIVENS: density of the oil = 0.9 g/mL each fatty acid side chain of the oil has 18 C atoms (C_18: the carbonyl carbon, plus a C_17 carbon chain). Assume an average of one unsaturation per side chain.arrow_forward
- TRUE OR FALSE SAPONIFICATION: I. Can be carried out with HCl II. NaOH undergoes hydrolysis III. Its principle is used to determine the number of milligrams of KOH required to neutralize the fatty acids from the complete hydrolysis of 1g of fat.arrow_forwardWhich compound will give a red color with Seliwanoff's reagent? glucitol starch glucose galactose sucrose fructosearrow_forward5. Order the following 4 fatty acids (labeled a through d) in order of increasing melting point (lowest melting point first, highest melting point last). (*See attached screenshot)arrow_forward
- 1a) Draw the structure of an Omega-5 fatty acid with an 14:2 designation. b) Provide a reaction for the partial hydrogenation of the fatty acid in Question 1(a). Is the product that forms still an Omega-5 fatty acid? Explain your answer.arrow_forward4-chloro-2-pentene has a double bond that can have either the E or The Z configuration and a sterogenic center that can have either the R or The S configuration. How many steroisomers are possible ? Draw the structure of each?arrow_forwardWrite down the reactions: D-Glucose + HNO3 →arrow_forward
- Carbohydrate Esterification What effects does the catalyst have on the product? What is the stoicheometry of the reaction?arrow_forwardWhy are bactoprenol C55 alcohols? Why not C30, C80?arrow_forward1. Give a general equation for the hydrolysis of the tannins that are esters of gallic acid and glucose. It is not necessary to balance this equation, but indicate the reactants and products by general structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY