
ORGANIC CHEMISTRY-OWL V2 ACCESS
8th Edition
ISBN: 9781305582422
Author: Brown
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.7, Problem AQ
Linoleic acid is shown below. What makes this fatty acid particularly susceptible to autoxidation?
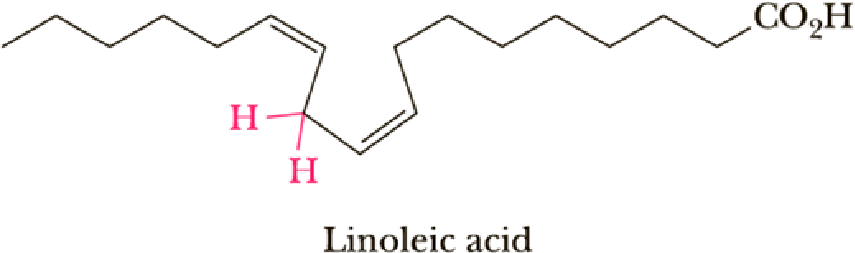
- 1. The red C—H bond has a low
bond dissociation energy because it is doubly allylic. - 2. The red C—H bond has a high bond dissociation energy because it is doubly allylic.
- 3. The red C—H bond is the most accessible to reaction with O2 because it is the least sterically crowded C—H bond.
- 4. Both 2 and 3.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The torsional energy in propane is 14 kJ/mol (3.4 kcal/mol). Because each H,H eclipsing interaction is worth 4.0 kJ/mol (1.0 kcal/mol) of destabilization, how much is one H,CH3 eclipsing interaction worth in destabilization?
Identify the alcohol reactant needed to produce each of the following compounds as the major
product of an alcohol dehydration reaction.
H,SO,
Alcohol
→ CH3–CH=CH–CH3
180°C
a.
H,SO,
Alcohol
→ CH,=CH-CH–CH3
180°C
CH3
b.
H,SO,
Alcohol
CH3-CH-CH2–0–CH,-CH–CH3
140°C
ČH3
с.
H,SO,
Alcohol
CH,=CH-CH,–CH3
180°C
H,SO,
Alcohol
CH;-C=C-CH3
180°C
CH3 CH3
e.
Oxaloacetic acid (or 2‑ketosuccinic acid) is a very important intermediate in metabolism. The compound is involved in the citric acid cycle for energy production within the cell. However, the compound is unstable and slowly decomposes spontaneously. Draw the decomposition products.
Chapter 8 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
Ch. 8.2 - Prob. 8.1PCh. 8.4 - Name and draw structural formulas for all...Ch. 8.4 - Using the table of bond dissociation enthalpies in...Ch. 8.5 - Prob. 8.4PCh. 8.6 - Given the solution to Example 8.5, predict the...Ch. 8.7 - Prob. 8.6PCh. 8.7 - Linoleic acid is shown below. What makes this...Ch. 8.7 - Prob. BQCh. 8.7 - Prob. CQCh. 8.7 - The strength of the HO bond in vitamin E is weaker...
Ch. 8.7 - Prob. EQCh. 8.8 - Prob. 8.7PCh. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10PCh. 8 - Prob. 8.11PCh. 8 - Account for the fact that among the chlorinated...Ch. 8 - Name and draw structural formulas for all possible...Ch. 8 - Prob. 8.14PCh. 8 - There are three constitutional isomers with the...Ch. 8 - Following is a balanced equation for bromination...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Cyclobutane reacts with bromine to give...Ch. 8 - Prob. 8.21PCh. 8 - Following is a balanced equation for the allylic...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - The major product formed when methylenecyclohexane...Ch. 8 - Prob. 8.26PCh. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Write the products of the following sequences of...Ch. 8 - Using your reaction roadmap as a guide, show...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - Prob. 8.34P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the product of intramolecular dehydration of pentanol-2 CH3CH( OH)CH2CH2CH3 ? a. Pentene-3b. Pentene-2c. Pentaned.Pentene-1arrow_forwardWhich of the following reactions is an example of an elimination reaction? A - CH CH₂CH₂CH₂OH CH CH₂CH₂ CH₂CH₂), NGOH CH₂CH.CHCH, NOOH SCHICH HO CH,arrow_forwardGive the major organic product for each step of the following reaction.arrow_forward
- a. In an aqueous solution, d-glucose exists in equilibrium with two six-membered ring compounds. Draw the structures of these compounds.b. Which of the six-membered ring compounds will be the major product?arrow_forwardIdentify the folowing. 1. When -OH cleaves in R-OH , alcohol reacts through ___ reaction. 2. The addition of an ] OH group to each of the two double-bond carbons. 3. It is the union of two aldehyde or ketone molecules to form β hydroxyl aldehydes or ketones. 4. The product of the reaction of isopropylbenzene with ________________ is methylphenyl ketone. 5. The product of the reaction of acetone (propanone) with bromine and ________ is 1-bromo-2-propanone. 6. The product of the reaction of propanamide with _______ is ethyl propanoate. 7. Aldehydes are easily oxidized to yield ________, but ketones are generally inert toward oxidation.arrow_forwardDraw a structural formula of the major alkene formed in each b-elimination.arrow_forward
- Give the major organic product for the reaction. CH₂ CH₂ CH₂ C CH 1. NaNH, 2. CH₂CH₂Clarrow_forwardDraw the structure(s) of the major organic product(s) of the following reaction. u + 1. Dry THF 2. aqueous NH Cl at 0°arrow_forwardThis image is the final product that is produced. If a synthesis started with benzene and other organic compounds (four carbons or less) what would the synthesis look like to achieve this final product.arrow_forward
- Draw the structure(s) of the major organic product(s) of the following reaction. معمر H + 1. Dry hexane 2, aqueous H₂SO, at oºarrow_forwardThis image is the final product that is produced. If a synthesis started with benzene and other organic compounds (four carbons or less) what would the synthesis look like to achieve this final product.arrow_forwardWhat is the product of this?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY