
Concept explainers
A 4-L pressure cooker has an operating pressure of 175 kPa. Initially, one-half of the volume is filled with liquid water and the other half by water vapor. The cooker is now placed on top of a 750-W electrical heating unit that is kept on for 20 min. Assuming the surroundings to be at 25°C and 100 kPa, determine (a) the amount of water that remained in the cooker and (b) the exergy destruction associated with the entire process.
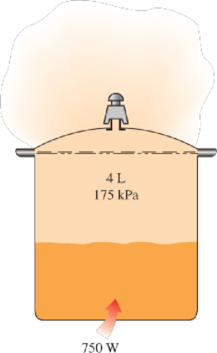
FIGURE P8–114
(a)
The final mass of water that remained in the cooker.
Answer to Problem 114RP
The final mass of water that remained in the cooker is
Explanation of Solution
Express the mass balance for a pressure cooker which acts as a system.
Here, initial mass is
Write an energy balance for a system.
Here, internal energy at state 1 and 2 is
Calculate the initial mass in the tank
Here, saturated liquid specific volume is
Calculate the initial internal energy
Here, specific internal energy of saturated fluid is
Calculate the initial entropy
Here, specific entropy of saturated fluid is
Write the internal energy at state 2
Here, dryness fraction at state 2 is
Write the specific volume at state 2
Write the formula to calculate the mass of the water remained in tank
Here, volume of the cooker is
Conclusion:
From the Table A-5 of “Saturated water: Pressure”, obtain the saturated liquid specific volume
From the Table A-5 of “Saturated water: Pressure”, obtain the enthalpy
Since one half of the volume is filled with liquid water and other half by water vapor, calculate the volume of tank
Substitute
Substitute 1.893 kg for
Substitute 1.893 kg for
Substitute
Substitute
Re-write the Equation (II) using the Equation (I),
Substitute
Substitute
Substitute 0.001918 for
Substitute
Thus, the final mass of water that remained in the cooker is
(b)
The amount of exergy destructed during the heating process.
Answer to Problem 114RP
The amount of exergy destructed during the heating process is
Explanation of Solution
For a closed system, write the simplification rate form of the entropy balance on an extended system which includes cooker and its immediate surroundings.
Here, entropy generation is
Calculate the exergy destroyed during the process
Here, dead state temperature is
Substitute Equation (XI) in Equation (XII).
Calculate the entropy at state 2
Conclusion:
Substitute 0.001918 for
Substitute 1.8945 kg for
Substitute 298 K for
Thus, the amount of exergy destructed during the heating process is
Want to see more full solutions like this?
Chapter 8 Solutions
Thermodynamics: An Engineering Approach
- A piston–cylinder device contains 8 kg of refrigerant134a at 0.7 MPa and 60°C. The refrigerant is now cooled at constant pressure until it exists as a liquid at 20°C. If the surroundings are at 100 kPa and 20°C, determine the exergy of the refrigerant at the initial and the final states.arrow_forwardRefrigerant-134a is to be compressed from 0.14 MPa and 10°C to 0.8 MPa and 50°C steadily by a compressor. Taking the environment conditions to be 20°C and 95kPa. Determine the EXERGY change of the refrigerant during this process and the minimum work input that needs to be supplied to the compressor per unit mass of the refrigerant. P.S please help me with this question, And please also show me your solution clearly. Thank you very much. Godblessarrow_forward8-90 Steam enters a turbine at 9 MPa, 600 degrees C, and 60 m/s and leaves at 20 kPa and 90 m/s with a moisture content of 5 percent. The turbine is not adequately insulated and it estimated that heat is lost from the turbine at a rate of 220 kW. The power output of the turbine is 4.5 MW. Assuming the surroundings to be at 25 degrees C, determine (a) the reversible power output of the turbine, (b) the exergy destroyed within the turbine, and (c) the second-law efficiency of the turbine. (d) Also, estimate the possible increase in the power output of the turbine if the turbine were perfectly insulated. (Please type answer no write by hend)arrow_forward
- A 4-L pressure cooker has an operating pressure of 175 kPa. Initially, one-half of the volume is filled with liquid water and the other half by water vapor. The cooker is now placed on top of a 750-W electrical heating unit that is kept on for 20 min. Assuming the surroundings to be at 25°C and 100 kPa, determine the exergy destruction associated with the entire process.arrow_forwardRefrigerant-134a at 140 kPa and 210C is compressed by an adiabatic 1.3-kW compressor to an exit state of 700 kPa and 60C. Neglecting the changes in kinetic and potential energies, determine (a) the isentropic efficiency of the compressor, (b) the volume flow rate of the refrigerant at the compressor inlet, in L/min, and (c) the maximum volume flow rate at the inlet conditions that this adiabatic 1.3-kW compressor can handle without violating the second law.arrow_forwardConsider a thermal energy reservoir at 1500 K that can supply heat at a rate of 150,000 kJ/h. Determine the exergy of this supplied energy, assuming an environment temperature of 25°C.arrow_forward
- Consider two geothermal wells whose energy contents are estimated to be the same. Will the exergies of these wells necessarily be the same? Explain.arrow_forwardSteam at 7 MPa and 400°C enters a two-stage adiabatic turbine at a rate of 15 kg/s. Ten percent of the steam is extracted at the end of the first stage at a pressure of 1.8 MPa for other use. The remainder of the steam is further expanded in the second stage and leaves the turbine at 10 kPa. If the turbine has an isentropic efficiency of 88 percent, determine the wasted power potential during this process as a result of irreversibilities. Assume the surroundings to be at 25°C.arrow_forwardConsider two systems that are at the same pressure as the environment. The first system is at the same temperature as the environment, whereas the second system is at a lower temperature than the environment. How would you compare the exergies of these two systems?arrow_forward
- Air enters the evaporator section of a window air conditioner at 100 kPa and 27°C with a volume flow rate of 6 m3 /min. Refrigerant-134a at 120 kPa with a quality of 0.3 enters the evaporator at a rate of 2 kg/min and leaves as saturated vapor at the same pressure. Determine the exit temperature of the air and the exergy destruction for this process, assuming heat is transferred to the evaporator of the air conditioner from the surrounding medium at 32°C at a rate of 30 kJ/min.arrow_forwardA piston–cylinder device contains 8 kg of refrigerant134a at 0.7 MPa and 60°C. The refrigerant is now cooled at constant pressure until it exists as a liquid at 20°C. If the surroundings are at 100 kPa and 20°C, determine the exergy destroyed during this process.arrow_forwardLong cylindrical steel rods (ρ = 7833 kg/m3 and cp = 0.465 kJ/kg·°C) of 10-cm diameter are heat-treated by drawing them at a velocity of 3 m/min through a 6-m-long oven maintained at 900°C. If the rods enter the oven at 30°C and leave at 700°C, determine the rate of exergy destruction associated with this heat transfer process. Take T0 = 25°C.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





