
a)
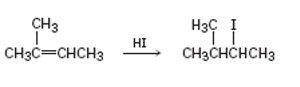
Interpretation:
The reaction given has a serious drawback. The potential problem in it is to be explained.
Concept introduction:
The addition of hydrogen halides to unsymmetrical
To explain:
The potential problem in the reaction given.
b)
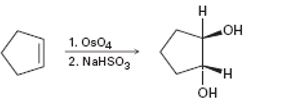
Interpretation:
The reaction given has a serious drawback. The potential problem in it is to be explained.
Concept introduction:
Alkenes undergo hydroxylation when treated first with OsO4 and then with NaHSO3. The reaction occurs with syn stereochemistry. Both –OH groups add to the double bond from the same face to give a cis-1,2-
To explain:
The potential problem in the reaction given.
c)
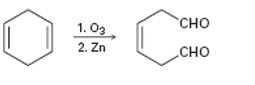
Interpretation:
The reaction given has a serious drawback. The potential problem in it is to be explained.
Concept introduction:
During ozonolysis ozone adds to the double bond in an alkene to give an ozonide. The ozonide on treatment with Zn in the presence of acid gets cleaved to yield carbonyl compounds as products. During the reaction each carbon in the double bond gets an oxygen atom.
To explain:
The potential problem in the reaction given.
d)
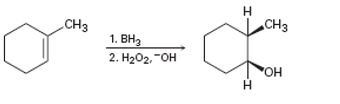
Interpretation:
The reaction given has a serious drawback. The potential problem in it is to be explained.
Concept introduction:
Alkenes can be hydrated using hydroboration-oxidation reaction. The reaction occurs with syn stereochemistry following anti markovnokov regiochemistry. The addition of both H and OH takes place from the same face of the double bond. The boron and hence the –OH group gets attached to less highly substituted carbon.
To explain:
The potential problem in the reaction given.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry
- You continue to impress people at the chemical company and they give you a special assignment. Compound C needs to be converted to Compound D and you are tasked to come up with a route for this. Design a route to take C to D, outlining the reagents you would use for each step. You should use only reactions that have been covered in the Oranic chem 1 course, and it will take more than one steparrow_forward2.Show the 2 step reaction Mechanisms of the compound belowarrow_forwardBased on your knowledge in biological chemistry reaction in biomolecules, what do you think is the best type and product for the above reaction? a. SN1 b. SN2 c. E1 d. E2 e. None of the abovearrow_forward
- V4 please solve the products for each below...arrow_forwardFill in necessary products reactants or reagants of these reactions. Please note the existence of enantionmers in some cases.arrow_forwardi need help filling out the following SN2 reactions with appopiate reactants, products, or reagents,arrow_forward
- Parsol®MCX (see structure below) is a popular UV B sunscreen used in many formulations. How would you have to modify the HWE Wittig reaction in order to synthesize this molecule?arrow_forwardHi there, can someone please help me solve this problem? Please make sure to clarify all steps taken to go about solving for the products and explain various terms and rules that should be known or correlate with the question. I'm using these questions to study for our final exam and need some extra clarification on rules and steps. Thank you very much in advance!arrow_forwardChemistry does methyl bromide favour SN1 or SN2 reactions or can be either depending on conditions ?arrow_forward
- You are planning to carry out a reaction between propyne, CH3C≡CH and sodium amide, NaNH2. You also need to choose an appropriate solvent for carrying out the reaction. Would ethanol be suitable for this purpose? Explain your rationale clearly.arrow_forwardFor question 2c below, which of the puzzle pieces makes the best sequence for each synthesis? You must solve the syntheses with the puzzle pieces provide and nothing else. One piece can only be used once.arrow_forwardHi there, can someone please help me solve for the products of the following reaction? Please make sure to clarify all steps taken to go about solving for the products and explain various terms and rules that should be known or correlate with the question. I'm using these questions to study for our final exam and need some extra clarification on rules and steps. Thank you very much in advance!arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

