
Concept explainers
Provide the IUPAC name for each of the following
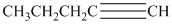
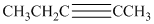
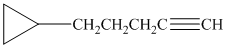
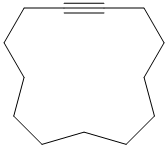
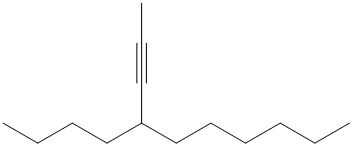
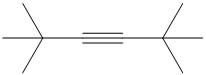
Interpretation:
IUPAC names for the given structures of alkyne are to be written.
Concept introduction:
The presence of a triple bond in a structure represents that the hydrocarbon is an alkyne.
To determine the name of the parent alkyne, select the longest continuous carbon chain containing the carbon‐carbon triple bond.
The parent name of the alkyne comes from the IUPAC name for the alkane having the same number of carbon atoms.
The numbers are assigned to the carbon atoms in the parent chain from the end which gives the lowest number to the substituents.
The name of any substituent atom or group and its location is indicated.
Name of substituent present in the structure is written before the name of the parent chain.
Answer to Problem 16P
Solution:
a)
b)
c)
d)
e)
f)
g)
Explanation of Solution
a) The structure is:
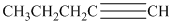
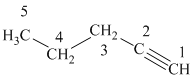
The given structure contains a triple bond. There are
b) The structure is:
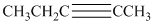
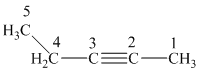
The given structure contains a triple bond. There are
Hence the name for the given structure is
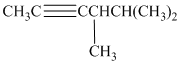
c) The structure is:
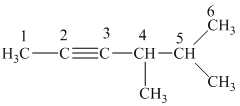
The longest chain in the given structure containing the triple bonded carbon atoms consists of
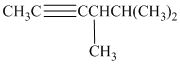
d) The structure is:
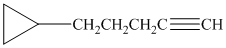
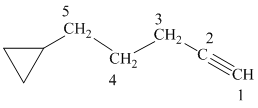
The longest chain in the given structure containing the triple bonded carbon atoms consists of
e) The structure is:
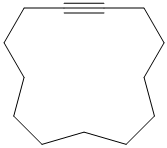
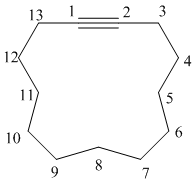
The longest chain in the given structure containing the triple bonded carbon atoms consists of
f) The structure is:
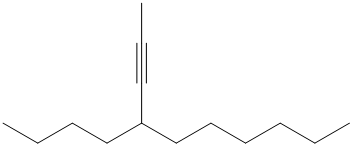
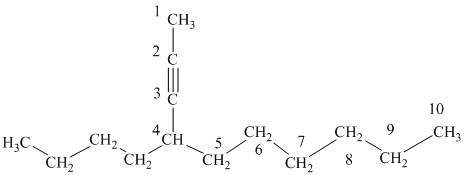
The longest chain in the given structure containing the triple bonded carbon atoms consists of
g) The structure is:
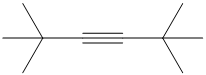
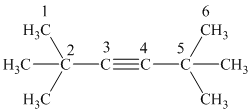
The longest chain in the given structure containing the triple bonded carbon atoms consists of
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry - Standalone book
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





