
Concept explainers
Interpretation:
The range of chemical shifts in the given compound is to be determined.
Concept introduction:
Nuclear magnetic resonance (NMR) is one of the most capable analytical techniques used for determining the functional groups and how the atoms are structured and arranged in a molecule.
A compound containing protons or carbon-13 when placed under very strong magnetic field and treated with
Nuclear magnetic resonance spectroscopy is a graph showing characteristic energy absorption frequencies and intensities of a compound under magnetic field.
Chemical shifts are positions of signals along the x-axis in the nuclear magnetic spectroscopy. It gives an idea on how many different hydrogen atoms are present in a given H-NMR for a particular compound.
More electronegative atoms attached to the group, more the protons are deshielded; more will be the chemical shift value.
Answer to Problem 1PP
Solution:
Chemical shifts for ethyl acetate are at
Explanation of Solution
Structure of ethyl acetate
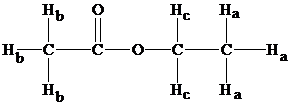
From the structure, we have three types of hydrogens and from table 9.1 chemical shifts result in the values as follows:
The chemical shifts are in the ranges of
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- Logically deduce the structure of compound 1B whose Spectra are given: Molecular formula C4H8Oarrow_forwardProvide your expectation of the chemical shifts of each molecule below in the 1H NMR spectrum of the molecules below. Please label the non-equivalent protons in each given molecule.arrow_forwardDraw the structure for a compound with the formula C8H10O3 that exihibits the 1H-NMR spectrum provided integral parts are also provided in a table above the 1H-NMR picture.arrow_forward
- Following are infrared spectra of nonane and 1-hexanol. Assign each compound its correct spectrum.arrow_forwardFollowing are two constitutional isomers with the molecular formula C4H8O2. (a) Predict the number of signals in the 1H-NMR spectrum of each isomer. (b) Predict the ratio of areas of the signals in each spectrum. (c) Show how you can distinguish between these isomers on the basis of chemical shift.arrow_forward3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the C1 vinylic proton (J = 16 Hz) and the C3 methylene protons (J = 8 Hz). Draw a tree diagram for the C2 proton signal, and account for the fact that a five-line multiplet is observed.arrow_forward
- Following is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122. Suggest a structure for this compound. (Data from http://webbook.nist.gov/chemistry/.)arrow_forwardDraw here the structure of 2-Chloropropene and encircle or point using arrows the different kinds of protons that would show in its 1H NMR spectrum.arrow_forwardThe following is the predicted 1H-NMR spectrum for an unknown compound with molecular formula C6H14O . This compound is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas.arrow_forward
- III) Can 1H NMR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. Predict the 1H NMR spectrum for each compound (include integration, multiplicity, and approximate chemical shift). For each set, be sure that you put them in a data table format!arrow_forwardNeed some assistance with NMR question please! Thank you so much! Part 3B Set 2. Can 1H NMR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. Predict the 1H NMR spectrum for each compound (include integration, multiplicity, and approximate chemical shift). Either draw the actual spectrum or put in a data table format.arrow_forwardPart 3B Set 1. Can 1H NMR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. Predict the 1H NMR spectrum for each compound (include integration, multiplicity, and approximate chemical shift). Put it in data table format.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,



