
Interpretation: The order of elution of a mixture of triphenylmethanol, biphenyl, benzoic acid, and methyl benzoate from an alumina column should be predicted.
Concept introduction: A separation technique used for the segregation of components of chemical mixture based on the difference between the adsorption of different components in stationary and mobile phase is called column chromatography.
It is used for purification or segregation of compounds. It consists of two phases namely:
1. Stationary phase (solid)
2. Mobile phase (generally liquid)
Silica gel is commonly used as the stationary phase.
Elution is the process of extraction of one component from another with the help of the solvent. Eluent is the carrier part of mobile phase. Eluate is the material that comes up through the chromatogram.
Answer to Problem 1Q
The order of elution of the compounds in the given mixture is,
Explanation of Solution
The structures of the given compounds are as follows:
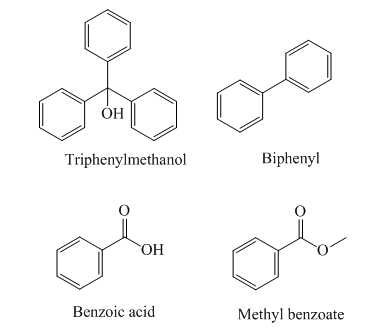
Triphenylmethanol has
The order of polarity of the given compounds is as follows:
Biphenyl is the least polar and therefore it is eluted first, followed by methyl benzoate, triphenylmethanol, and benzoic acid.
So the order of elution of the mixture of the given compounds is as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
Macroscale and Microscale Organic Experiments
- How would the Rf values change if you used pure dichloromethane (no methanol) as the mobile phase for theTLC separation of curcumin? What if you used pure methanol (no dichloromethane)?arrow_forward(h) Describe a chromatographic technique that could be suitable for studying the molecular weight distribution of poly(phenylenevinylene) derivatives that are soluble in organic solvents. Explain the physical basis on which separation could occur and suggest how the polymers could be detected after elution.arrow_forward(Column Chromatography) What complications would a dried-out column (solvent level is below the top of the silica) introduce to the elution and isolation of pigments?arrow_forward
- The particle size of the silica gel influences the quality of the separation in column chromatography. Please explain this effect.arrow_forwardWhat are the units of the retention factor (Rf)? Briefly explain your conclusion.arrow_forwardWhat is the order in which the following compounds would he eluted from an HPLC column containing a reversed -phase packing?(a) benzene, diethyl ether, n-hexane(b) acetone, dichloroethane, acetamidearrow_forward
- Predict the order of elution for the silica gel adsorption TLC separation of the mixture containing aspirin, acetaminophen and caffeine using methylene chloride solvent. Explain.arrow_forwardA neutral compound has a partition coefficient of 5 5 between ether and water. What percentage of the compound would be extracted from 10 mL of water if 10 mL of ether were used to extract the compound?arrow_forwardDescribe how to prepare adulterated palm oil samples before analysing it with Raman spectrometer. Also the describe the process how the Raman spectrometer will analyze the adulterated palm oil samplesarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


