
Interpretation:
Temperature condition is needed to be explained in the given dehydrogenation reaction.
Concept introduction:
Dehydrogenation: dehydrogenation is a process of elimination of hydrogen molecule from the substrate at high temperature condition.
Free energy: reaction is feasible when free energy is negative.
Answer to Problem 48PP
Reaction is feasible at higher temperature conditions only since
Explanation of Solution
To explain: the conditions for the given reaction.
Given reaction is shown below.
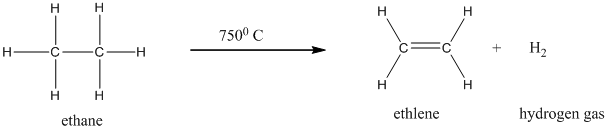
Clear from the reaction that the ethane molecule converted ethylene molecule at high temperature condition by releasing hydrogen gas.
Given reaction refers the dehydrogenation process at high temperatures. Given substrate is ethane and this ethane molecule heated at high temperature (7500 C) to produce ethylene and hydrogen gas.
To give: the reason for high temperatures needed for the dehydrogenation process.
Given reaction is favorable when
At higher temperature condition, the given reaction is feasible since
Conclusion
At higher temperature conditions, value of free energy will be negative so the dehydrogenation reaction is feasible at higher temperature conditions.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





