
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Chapter 9, Problem 4RQ
Interpretation Introduction
Interpretation:
Differences between crystalline solid and amorphous solid, ionic solid and molecular solid, molecular solid and network solid, metallic solid and network solid have to be explained.
Concept introduction:
- Solids constitute the major part of the matter in the universe. Beneath the earth and above the sky in our Universe, as well as in our everyday life, solids can be found. Solids do have such a profound significance since the beginning of the Universe and human evolution.
- A solid is anything that is firm and stable in shape.
Physics and Chemistry do provide clear cut explanation for the structure of solids. The firm and dense nature of solids is due to the strong intermolecular forces between their components which are nothing but molecules or ions.
- On the basis of the arrangement of the components of a solid, there are two distinct types of solids viz., crystalline solids and amorphous solids. These two types differ in the arrangement of their respective components and so in their properties.
- Crystalline solids have their own sub-classification. The types of solids can be summarized as follows –
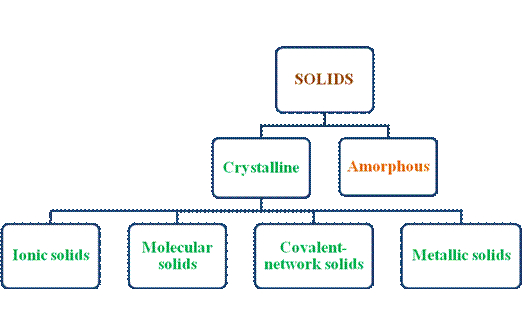
- Crystalline solids have well-defined regular, compact, orderly arrangements of their components in a very long range order. They are termed as true solids. Amorphous solids lack such well defined arrangement of its components thus disordered or random arrangement does exist in them. They are termed as pseudo solids or super cooled liquids.
- Ionic solids, molecular solids, covalent solids and metallic solids are the types of crystalline solids. The components that are found to be arranged in regular, compact, three dimensional patterns are ions, then it is an ionic solid.
- If molecules are arranged in such a fashion, then it is molecular solid whereas metal atoms are arranged in such a manner, it is metallic solid. In Covalent solids the components are atoms bonded by covalent bond repetitively and thus forms huge network form of solid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Chemistry: An Atoms First Approach
Ch. 9 - What are intermolecular forces? How do they differ...Ch. 9 - Define the following terms and describe how each...Ch. 9 - Compare and contrast solids, liquids, and gases.Ch. 9 - Prob. 4RQCh. 9 - What is a lattice? What is a unit cell? Describe a...Ch. 9 - What is closest packing? What is the difference...Ch. 9 - Describe, in general, the structures of ionic...Ch. 9 - Prob. 9RQCh. 9 - Prob. 10RQCh. 9 - Compare and contrast the phase diagrams of water...
Ch. 9 - It is possible to balance a paper clip on the...Ch. 9 - Prob. 2ALQCh. 9 - Prob. 3ALQCh. 9 - Prob. 4ALQCh. 9 - Prob. 5ALQCh. 9 - Prob. 6ALQCh. 9 - Prob. 7ALQCh. 9 - Prob. 8ALQCh. 9 - Prob. 9ALQCh. 9 - Prob. 10ALQCh. 9 - Prob. 11ALQCh. 9 - Prob. 12QCh. 9 - In the diagram below, which lines represent the...Ch. 9 - Prob. 14QCh. 9 - Atoms are assumed to touch in closest packed...Ch. 9 - Define critical temperature and critical pressure....Ch. 9 - Prob. 17QCh. 9 - Prob. 18QCh. 9 - Prob. 19QCh. 9 - Prob. 20QCh. 9 - Prob. 21QCh. 9 - A common response to hearing that the temperature...Ch. 9 - Prob. 23QCh. 9 - Prob. 24QCh. 9 - When wet laundry is hung on a clothesline on a...Ch. 9 - Cake mixes and other packaged foods that require...Ch. 9 - You have three covalent compounds with three very...Ch. 9 - Prob. 28QCh. 9 - Compare and contrast the structures of the...Ch. 9 - Prob. 30QCh. 9 - How could you tell experimentally if TiO2 is an...Ch. 9 - A common prank on college campuses is to switch...Ch. 9 - A plot of In (Pvap) versus 1/T (K) is linear with...Ch. 9 - Prob. 34QCh. 9 - Identify the most important types of interparticle...Ch. 9 - Prob. 36ECh. 9 - Predict which substance in each of the following...Ch. 9 - Consider the compounds CI2, HCI. F2, NaF, and HF....Ch. 9 - Prob. 39ECh. 9 - Consider the following electrostatic potential...Ch. 9 - In each of the following groups of substances,...Ch. 9 - Prob. 42ECh. 9 - The shape of the meniscus of water in a glass tube...Ch. 9 - Prob. 44ECh. 9 - Prob. 45ECh. 9 - Prob. 46ECh. 9 - X rays from a copper X-ray tube ( = 154 pm) were...Ch. 9 - The second-order diffraction (n = 2) for a gold...Ch. 9 - A topaz crystal has an interplanar spacing (d) of...Ch. 9 - X rays of wavelength 2.63 were used to analyze a...Ch. 9 - Prob. 51ECh. 9 - Prob. 52ECh. 9 - Prob. 53ECh. 9 - Iridium (Ir) has a face-centered cubic unit cell...Ch. 9 - You are given a small bar of an unknown metal X....Ch. 9 - A metallic solid with atoms in a face-centered...Ch. 9 - Titanium metal has a body-centered cubic unit...Ch. 9 - Barium has a body-centered cubic structure. If the...Ch. 9 - The radius of gold is 144 pm, and the density is...Ch. 9 - The radius of tungsten is 137 pm and the density...Ch. 9 - What fraction of the total volume of a cubic...Ch. 9 - Iron has a density of 7.86 g/cm3 and crystallizes...Ch. 9 - Prob. 63ECh. 9 - Prob. 64ECh. 9 - Selenium is a semiconductor used in photocopying...Ch. 9 - Prob. 66ECh. 9 - Prob. 67ECh. 9 - Prob. 68ECh. 9 - The structures of some common crystalline...Ch. 9 - The unit cell for nickel arsenide is shown below....Ch. 9 - Cobalt fluoride crystallizes in a closest packed...Ch. 9 - The compounds Na2O, CdS, and ZrI4. all can be...Ch. 9 - What is the formula for the compound that...Ch. 9 - Prob. 74ECh. 9 - A certain metal fluoride crystallizes in such a...Ch. 9 - The structure of manganese fluoride can be...Ch. 9 - The unit cell of MgO is shown below l Does MgO...Ch. 9 - In solid KCl the smallest distance between the...Ch. 9 - The CsCl structure is a simple cubic array of...Ch. 9 - MnO has either the NaCI type structure or the CsCI...Ch. 9 - Prob. 81ECh. 9 - What type of solid will each of the following...Ch. 9 - The memory metal, nitinol, is an alloy of nickel...Ch. 9 - Superalloys have been made of nickel and aluminum....Ch. 9 - Perovskite is a mineral containing calcium,...Ch. 9 - A mineral crystallizes in a cubic closest packed...Ch. 9 - Materials containing the elements Y, Ba, Cu, and O...Ch. 9 - The structures of another class of ceramic,...Ch. 9 - Plot the following data and determine Hvap for...Ch. 9 - From the following data for liquid nitric acid,...Ch. 9 - Prob. 91ECh. 9 - Prob. 92ECh. 9 - Prob. 93ECh. 9 - Diethyl ether (CH3CH2OCH2CH3) was one of the first...Ch. 9 - A substance, X, has the following properties:...Ch. 9 - Use the heating-cooling curve below to answer the...Ch. 9 - The molar heat of fusion of sodium metal is 2.60...Ch. 9 - Prob. 98ECh. 9 - What quantity of energy does it take to convert...Ch. 9 - Consider a 75.0-g sample of H2O(g) at 125C. What...Ch. 9 - An ice cube tray contains enough water at 22.0C to...Ch. 9 - A 0.250-g chunk of sodium metal is cautiously...Ch. 9 - Prob. 103ECh. 9 - Prob. 104ECh. 9 - Prob. 105ECh. 9 - Prob. 106ECh. 9 - Prob. 107ECh. 9 - Consider the following data for xenon: Triple...Ch. 9 - Some of the physical properties of H2O and D2O are...Ch. 9 - Rationalize the following boiling points:Ch. 9 - Prob. 111AECh. 9 - Consider the following enthalpy changes:...Ch. 9 - Prob. 113AECh. 9 - Boron nitride (BN) exists in two forms. The first...Ch. 9 - Prob. 115AECh. 9 - Argon has a cubic closest packed structure as a...Ch. 9 - Prob. 117AECh. 9 - A 20.0-g sample of ice at 10.0C is mixed with...Ch. 9 - In regions with dry climates, evaporative coolers...Ch. 9 - The critical point of NH3 is 132C and 111 atm, and...Ch. 9 - Which of the following compound(s) exhibit only...Ch. 9 - Which of the following statements about...Ch. 9 - Prob. 123CWPCh. 9 - Aluminum has an atomic radius of 143 pm and forms...Ch. 9 - Pyrolusite is a mineral containing manganese ions...Ch. 9 - The structure of the compound K2O is best...Ch. 9 - Prob. 127CWPCh. 9 - Some ice cubes at 0c with a total mass of 403 g...Ch. 9 - The enthalpy of vaporization for acetone is 32.0...Ch. 9 - Prob. 130CWPCh. 9 - When I mole of benzene is vaporized at a constant...Ch. 9 - Prob. 132CPCh. 9 - Using the heats of fusion and vaporization for...Ch. 9 - Prob. 134CPCh. 9 - Consider two different organic compounds, each...Ch. 9 - Prob. 136CPCh. 9 - Prob. 137CPCh. 9 - Prob. 138CPCh. 9 - Prob. 139CPCh. 9 - Prob. 140CPCh. 9 - Mn crystallizes in the same type of cubic unit...Ch. 9 - Prob. 142CPCh. 9 - Some water is placed in a sealed glass container...Ch. 9 - The molar enthalpy of vaporization of water at 373...Ch. 9 - Prob. 145CPCh. 9 - Rubidium chloride has the sodium chloride...Ch. 9 - Prob. 147IPCh. 9 - A metal burns in air at 600c under high pressure...Ch. 9 - Prob. 149IPCh. 9 - General Zod has sold Lex Luthor what Zod claims to...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Silicon carbide, SiC, is a very hard, high-melting solid. What kind of crystal forces account for these properties?arrow_forwardAn amorphous solid can sometimes be converted to a crystalline solid by a process called annealing. Annealing consists of heating the substance to a temperature just below the melting point of the crystalline form and then cooling it slowly. Explain why this process helps produce a crystalline solid.arrow_forwardAt very low temperatures oxygen, O2, freezes and forms a crystalline solid. Which best describes these crystals? (a) ionic (b) covalent network (c) metallic (d) amorphous (e) molecular crystalsarrow_forward
- How do ionic solids differ in structure from molecular solids? What are the fundamental panicles in each? Give two examples of each type of solid and indicate the individual particles that make up the solids in each of your examples.arrow_forwardA pure substance X has the following properties: Mp=90C, increasing slightly as pressure increases; normal bp=120C; liquid vp=65mm Hg at 100C, 20 mm Hg at the triple point. (a) Draw a phase diagram for X. (b) Label solid, liquid, and vapor regions of the diagram. (c) What changes occur if, at a constant pressure of 100 mm Hg, the temperature is raised from 100C to 150C?arrow_forwardConsider the following data for xenon: Triple point: 121C, 280 torr Normal melting point: 112C Normal boiling point: 107C Which is more dense, Xe(s) or Xe(l)? How do the melting point and boiling point of xenon depend on pressure?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning