
(a)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(a)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
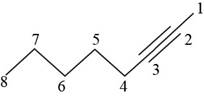
Figure 1
The structural formula for
(b)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(b)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
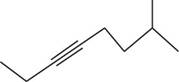
Figure 2
The structural formula for
(c)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(c)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
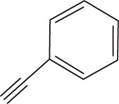
Figure 3
The structural formula for
(d)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(d)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
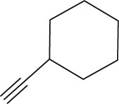
Figure 4
The structural formula for
(e)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(e)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
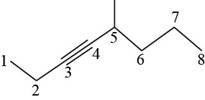
Figure 5
It is conferred form the above structure that the structural formula for
(f)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(f)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
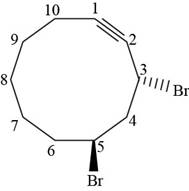
Figure 6
The stereochemistry along with structural formula for
(g)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(g)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
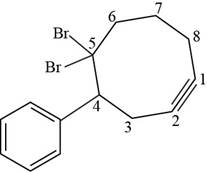
Figure 7
The structural formula for
(h)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(h)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
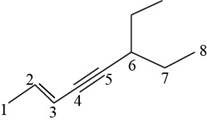
Figure 8
The structural formula for
(i)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(i)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
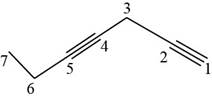
Figure 9
The structural formula for
(j)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(j)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
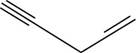
Figure 10
The structural formula for
(k)
To determine: The structural formula for
Interpretation: The structural formula for
Concept introduction: Structural formulas are used to describe the arrangement of atoms, groups or substituents in a molecule, whereas molecular formula describes the total number and the type of atoms present in a molecule. The chemical structures are described by IUPAC name or common names. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules.
(k)
Answer to Problem 9.26SP
The structural formula for
Explanation of Solution
The structural formula for
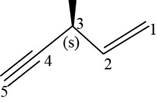
Figure 11
The structural formula for
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
- Following one which has the lowest molecular weight? and why? Perfluorocarbons, Polytetrafluoroethylene , polyvinyl fluoride, polyvinylidene fluoridearrow_forwardExplain why Hex–2-ene produces more soot than Hexanewhen combusted. Write this equation.arrow_forwardUsing your knowledge, explain why 1- dimethyl 2- bromopropane is difficult to purify using 2-methyl-2-butene as the starting compound. Use structures in your answer.arrow_forward
- Show the possible products of monochlorination of propane?arrow_forwardWhat are synthetic polymers?arrow_forwardChemistry Bromination of Stilbene In this experiment, we were going to brominate the carbon-carbon double bond of stilbene. The reaction conditions we were going to use to perform this reaction are considered “green” Describe why the procedure is “green.” Describe briefly how the Br2 adds to the double bond. Is the addition of Br2 to stilbene (or any alkene for that matter) anti-addition or syn-addition? What is the three-member ring that forms between the alkene and the Br2 called? Describe the differences between the Infrared spectra between the stilbene and the brominated product. Will the products that are produced be enentiomers of each other? Diastereomers of each other?arrow_forward
- Explain about Synthetic Polymers?arrow_forwardThe chemical tests that are used to classify hydrocarbons based on the type of bonding between carbon atoms are_____. Select all that apply. ____bromination (reaction with Br2) to form an alkyl halide _____oxidation with dilute or alkaline solutions of KMnO4 to form diol, ______bromination (reaction with Br2) to form a dihalide ______addition reaction with cold concentrated sulfuric acid, to form alkyl sulfonic acids which are soluble in H2SO _____addition reaction with cold concentrated sulfuric acid, to form alkyl sulfonic acids which are insoluble in H2SO4, and so form precipitate, which can be filtered ____oxidation with dilute or alkaline solutions of KMnO4 to form an alcoholarrow_forwardHow are these vehicles made from benzene?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


