
Draw a structural formula for the major organic product of each reaction and specify the most likely mechanism by which each is formed.
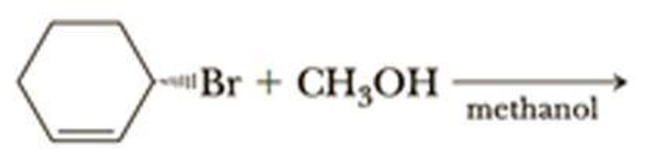
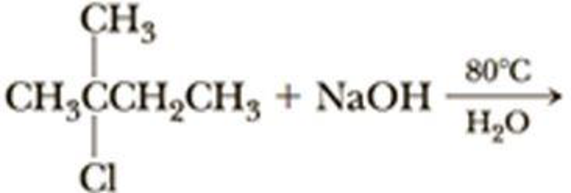
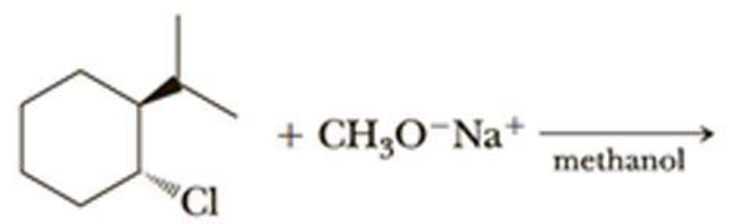
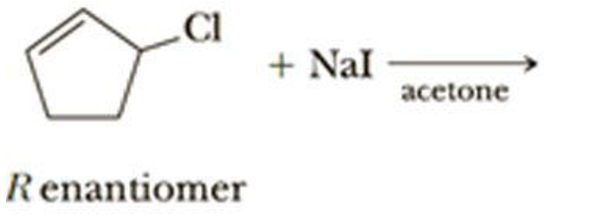
(g)
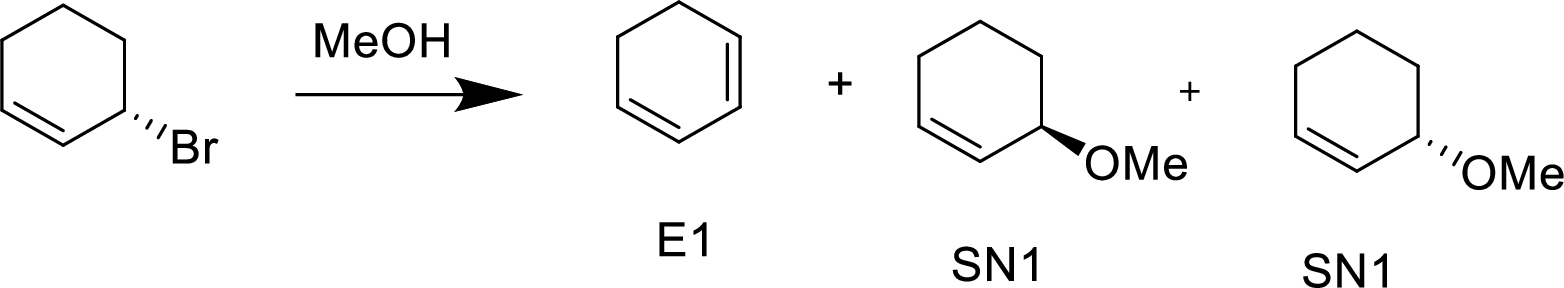
(a)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
Methanol is a polar protic solvent, moderate nucleophile and alkyl halide forms allyl cation, it is stable cation.
Hence, the given reaction is takes place in both
(b)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
The given alkyl halide is a tertiary halide, sodium hydroxide is strong base and water is polar solvent hence, the given reaction is takes place in
(c)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
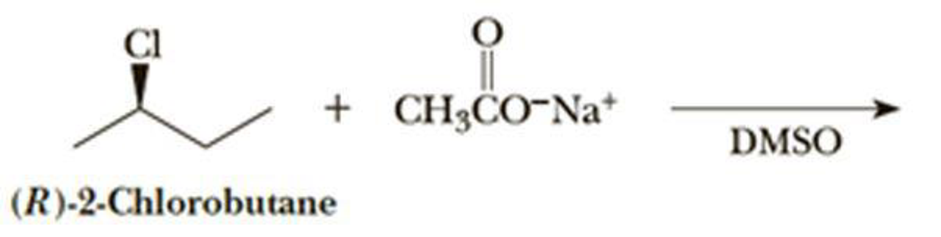
The given alkyl halide is a secondary halide, sodium acetate is strong neucleophile and DMSO is polar aprotic solvent hence, the given reaction is takes place in
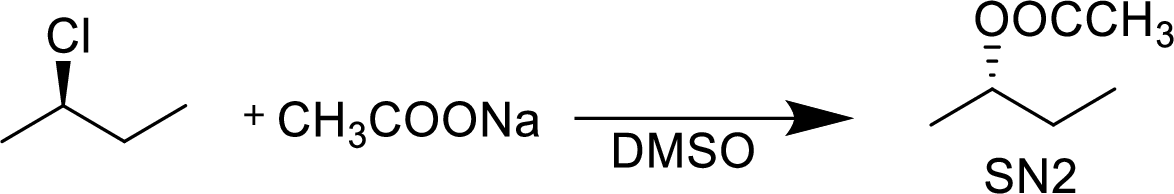
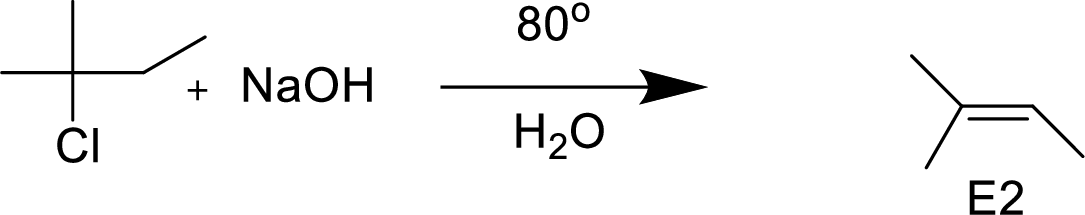
(d)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
The given alkyl halide is a secondary halide, sodium methoxide is strong base and methonal is polar solvent hence, the given reaction is takes place in
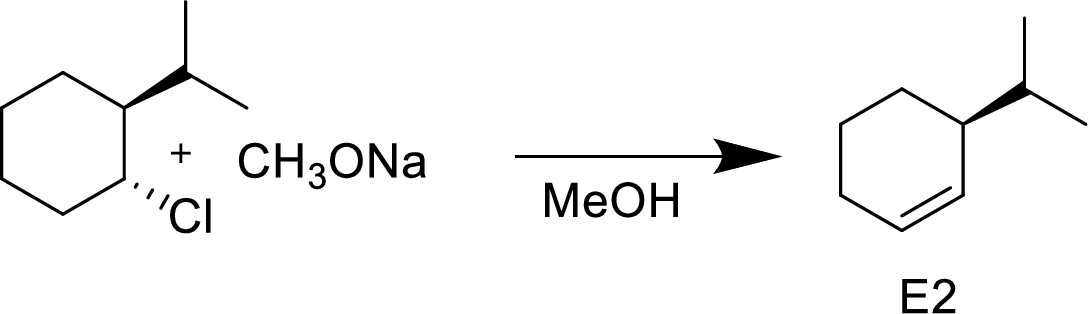
(e)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
The given alkyl halide is a secondary halide, iodide is strong nucleophile and acetone is polar solvent hence, the given reaction is takes place in
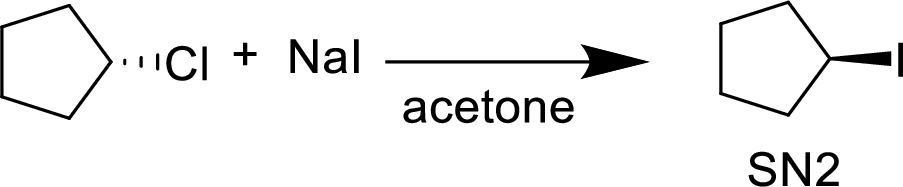
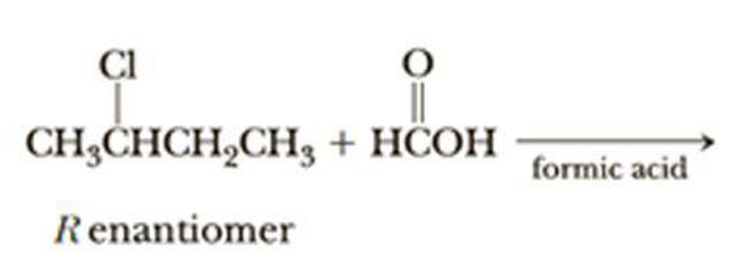
(f)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
The given alkyl halide is a secondary halide, formic acid is moderate nucleophile and is polar solvent hence, the given reaction is takes place in both
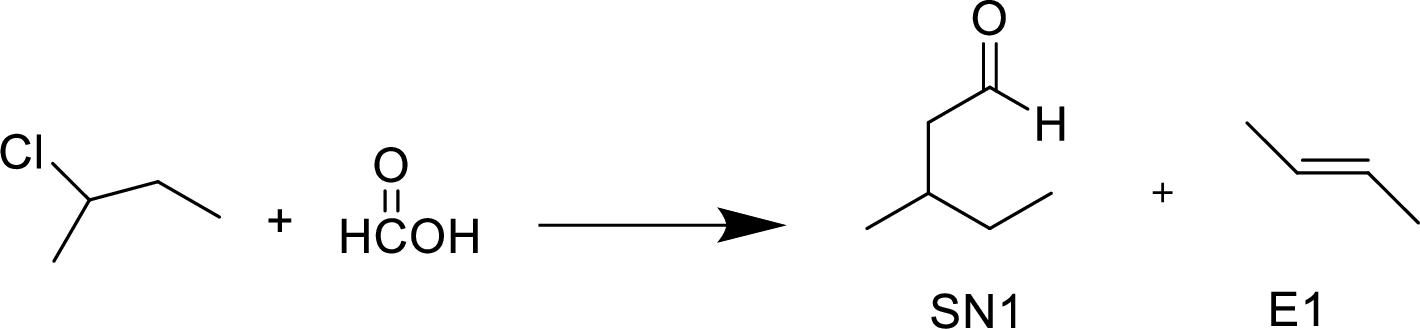
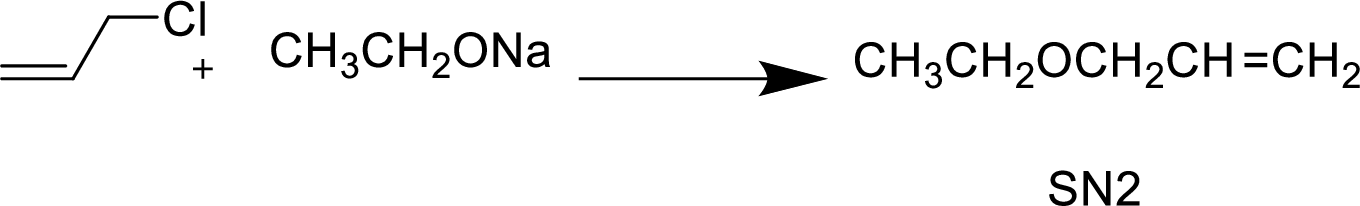
(g)
Interpretation:
The structural formula for the major organic product has to be drawn and mechanism of the given reaction has to be specified.
Concept Introduction:
The rate of the reaction is depends on a single reactant in reaction is known as
The first step of
Frist step is the slow step also rate determining step so the rate of the reaction is depends on the concentration of substrate only.
Nucleophile attacks the both front and back side of carbocation in
Order of the substrate that favored in
Elimination:
An atom or group are removed from saturated compound to give unsaturated alkene is known as elimination reaction.
In elimination, the removal of halogen ion forms a carbocation followed by removal of hydrogen ion forms an alkene is known as E1 reaction.
The abstraction of proton and removal of leaving group takes simultaneously means it is E2 reaction because the rate of reaction depends on both base and substrate.
E1 elimination fallows saytzeff rule (more substituted alkene is formed).
Explanation of Solution
The given alkyl halide is a allyl halide, sodium ethoxide is strong base and ethanol is polar solvent hence, the given reaction is takes place in
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- Describe the reaction mechanism for the hydrolysis of 2-bromo-2-methylpropane [CH3C(CH3)2Br] with aqueous hydroxide ions (OH-).arrow_forwardCHOOSE THE LETTER OF THE CORRECT ANSWER. What is the type of hybridization of the carboxyl carbon in the mechanism? Reaction mechanisms of organic molecules (alcohol to carboxylic acid) a. sp² b. sp¹‧⁵ c. sp³ d. sparrow_forwardIdentify the type of substitution mechanism (SN1, SN2) involved in the conversion of the alcohol shown into the corresponding alkyl halide.arrow_forward
- Which of the following reaction may have a different reaction mechanism compared to the others? a. Hydration b. Hydrohalogenation c. Halogenation d. Ether formationarrow_forwardPredict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely.(a) 1@bromohexane + sodium ethoxide in ethanol(b) 2@chlorohexane + NaOCH3 in methanolarrow_forwardWhat will be the resulting organic products’ structure when the following reactions occur and determine the mechanism (SN1, SN2, E1 or E2)? 1-bromobutane reacts with Potassium Hydroxidearrow_forward
- 1-Suggest a mechanism by which the ozone, O3 is being destructed by the presence of oxygen molecule, O2, and the sun light. 2-Methyl chloride, CH3Cl, is another molecule that destructs the ozone layer. Suggest a mechanism for the destruction of ozone by methyl chloride and the sun light.arrow_forwardChemical Reactions and Mechanisms: Use the space below for reactions and mechanisms. Initiation Propagation Termination (3) Toluene 1° Benzylicarrow_forwardPredict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely. isobutyl iodide + KOH in ethanol>waterarrow_forward
- Predict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely.(a) 1@bromohexane + sodium ethoxide in ethanoarrow_forwardPredict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely.(a) 1@bromohexane + sodium ethoxide in ethanolarrow_forwardExplain the mechanism of the following reaction using photodissociation: HCOOCH3 + hv -> CO + CH3OHarrow_forward
