
Concept explainers
a)
The thermal efficiency of an ideal diesel cycle using constant specific heats.
a)
Answer to Problem 161RP
The thermal efficiency of ideal diesel cycle is
Explanation of Solution
Draw
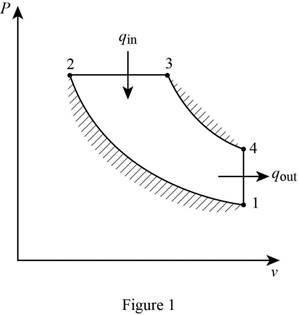
Assuming constant specific heats
Write the temperature and specific volume relation for the isentropic compression process 1-2.
Here, the specific heat ratio is
Write the ideal gas relation for the constant pressure heat addition process 2-3.
For the process 2-3,
Here, temperature at state 3 is
Write the expression of heat input to the cycle
Here, the specific heat at constant pressure is
Write the temperature and specific volume relation for isentropic expansion process 3-4.
Write the expression of heat rejected for constant volume heat rejection process 4-1
Here, specific heat at constant volume is
Write the expression to calculate the net work output of the engine
Write the expression of thermal efficiency of the ideal diesel cycle
Conclusion:
From Table A-2a, “Ideal-gas specific heats of various common gases”, obtain the following properties of air at room temperature.
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the thermal efficiency of an ideal diesel cycle is
b)
The thermal efficiency of ideal diesel cycle using variable specific heats.
b)
Answer to Problem 161RP
The thermal efficiency of an ideal diesel cycle is
Explanation of Solution
Assuming variable specific heats
Write the specific volume and relative specific volume relation for the isentropic compression process 1-2.
Here, the compression ratio is r, relative specific volume at state 1 is
Write the pressure, temperature, and specific volume relation for isentropic compression process 2-3.
For process 2-3,
Write the expression of heat addition for constant pressure heat addition process 2-3
Write the specific volume and relative specific volume relation for the isentropic expansion process 3-4.
Here, relative specific volume at state 4 is
Write the expression of heat rejected for constant volume heat rejection process 4-1
Write the expression of thermal efficiency of an deal diesel cycle
Conclusion:
Refer Table A-17, “Ideal gas properties of air”, obtain the following properties of air at temperature
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at
Substitute
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at
Substitute
Substitute
Thus, the thermal efficiency of an ideal diesel cycle is
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- An Otto cycle with a compression ratio of 8 begins its compression at 94 kPa and 10°C. The maximum cycle temperature is 900°C. Utilizing air-standard assumptions, determine the thermal efficiency of this cycle using variable specific heatsarrow_forwardConsider the ideal Otto, Stirling, and Carnot cycles operating between the same temperature limits. How would you compare the thermal efficiencies of these three cycles?arrow_forwardConsider an ideal gas-turbine cycle with two stages of compression and two stages of expansion. The pressure ratio across each stage of the compressor and turbine is 3. The air enters each stage of the compressor at 300 K and each stage of the turbine at 1200 K. Determine the back work ratio and the thermal efficiency of the cycle, assuming no regenerator is used.arrow_forward
- A regenerator of 75 percent effectiveness is used in an air-standard Brayton cycle working between pressures of 15 psia and 75 psia. Determine the work per pound of air and the efficiency of the cycle if the maximum and minimum temperatures of the cycles are 1700 R and 550 R, respectively.arrow_forwardHow do the inefficiencies of the turbine and the compressor affect the thermal efficiency of a gas-turbine engine?arrow_forwardIn an ideal Otto cycle, the initial pressure and temperature of air are 100 kPa and 18 degree C. Determine the maximum pressure in the cycle if the maximum temperature in the cycle is 600 deg C, and the compression ratio is 8.arrow_forward
- What is the cutoff ratio? How does it affect the thermal efficiency of a Diesel cycle?arrow_forwardWhy is the Carnot cycle not suitable as an ideal cycle for all power-producing cyclic devices?arrow_forwardIn an air standard gas turbine cycle, air at 14.5 psia and 70 F is first compressed to 80 psia in a compressor of 82 percent efficiency. The hot air leaving the combustion chamber at 1250 F is expanded back to 14.5 psia in a turbine of 85 percent efficiency. If a regenerator is inserted into the cycle to heat the air leaving the compressor to 650 F, determine the thermal efficiency of the cycle and the effectiveness of the regenerator.arrow_forward
- Air is used as the working fluid in a simple ideal Brayton cycle that has a pressure ratio of 12, a compressor inlet temperature of 300 K, and a turbine inlet temperature of 1000 K. Determine the required mass flow rate of air for a net power output of 70 MW, assuming both the compressor and the turbine have an isentropic efficiency of 85 percent. Assume constant specific heats at room temperature.arrow_forwardWhy are the back work ratios relatively high in gas-turbine engines?arrow_forwardAn ideal diesel engine has a compression ratio of 20 and uses air as the working fluid. The state of air at the beginning of the compression process is 95 kPa and 20°C. If the maximum temperature in the cycle is not to exceed 2200 K, determine the net work of the system as a function of compression ratio, r.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





