
Concept explainers
(a)
Interpretation: The structure for given NMR spectra should be predicted.
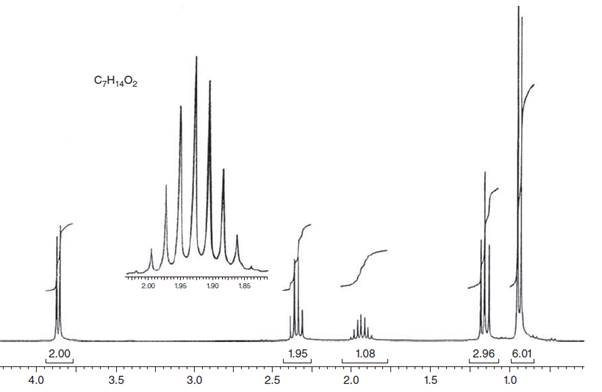
Concept Introduction:
NMR stands for nuclear magnetic resonance. A given compound contains different types of H-signals due to the presence of different magnetic environment within a molecule.
The number of signals obtained by the proton can be determined by spitting rule (n + 1) where n represents the number of the adjacent protons.
(b)
Interpretation: The structure for given NMR spectra should be predicted.
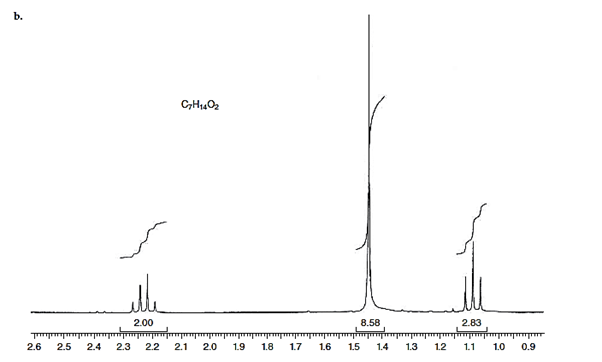
Concept Introduction:
NMR stands for nuclear magnetic resonance. A given compound contains different types of H-signals due to the presence of different magnetic environment within a molecule.
The number of signals obtained by the proton can be determined by spitting rule (n + 1) where n represents the number of the adjacent protons.
(c)
Interpretation: The structure for given NMR spectra should be predicted.
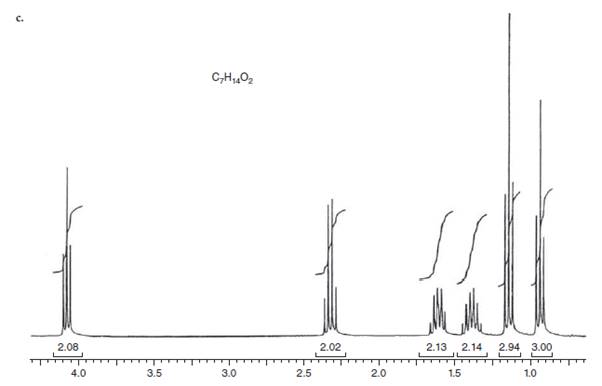
Concept Introduction:
NMR stands for nuclear magnetic resonance. A given compound contains different types of H-signals due to the presence of different magnetic environment within a molecule.
The number of signals obtained by the proton can be determined by spitting rule (n + 1) where n represents the number of the adjacent protons.
Trending nowThis is a popular solution!

Chapter 95 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Propose a structural formula for the analgesic phenacetin, molecular formula C10H13NO2, based on its 1H-NMR spectrum.arrow_forwardFollowing is the 1H-NMR spectrum of compound O, molecular formula C7H12. Compound O reacts with bromine in carbon tetrachloride to give a compound with the molecular formula C7H12Br2. The 13C-NMR spectrum of compound O shows signals at d 150.12, 106.43, 35.44, 28.36, and 26.36. Deduce the structural formula of compound O.arrow_forwardPropose a structure for an alcohol with molecular formula C5H10O by interpreting the following IR, 1H and 13C NMR.arrow_forward
- An aromatic compound K, whose molecular formula is C8H11N, is examined in the laboratory to elucidate its structure. The following observations were made: A) Compound K is soluble in dilute hydrochloric acid but insoluble in sodium hydroxide solution. B) Treatment of compound K with excess potassium hydroxide and benzenesulfonyl chloride, C(6)H(5)SO(2)Cl, results in the formation of a heterogeneous mixture. The NMR spectrum of compound K is shown below. C) Compound K when treated with acetic anhydride[CH3-C(O)-O-C(O)-CH3], gives compound L, whose molecular formula is C(10)H(13)ON. Compound L is insoluble in dilute acid or dilute base at room temperature, heating compound L in dilute acid or base, however, regenerates compound K. D) When compound L is heated with a mixture of concentrated nitric acid and sulfuric acid, a single product, compound M, with the molecular formula C(10)H(12)O(3)N(2) is formed in excellent yields. On the basis of these observations draw the structures of…arrow_forwardThe only organic compound obtained when compound Z undergoes the following sequence of reactions gives the 1H NMR spectrum shown. Identify compound Z.arrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.92 (t, 7.8 Hz, 3 H), 1.20 (s, 6H), 1.49 (q, 7.8 Hz, 2H), 1.85 (s, 1H) ppm Isomer B: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forward
- A compound reacts with methylmagnesium bromide followed by acidification to form the product with the following 1H NMR spectrum. Identify the compound.arrow_forwardFully interpret the 1H-NMR spectrum of 4-vinylbenzoic acid providedarrow_forwardA hydrocarbon, compound B, has molecular formula C6H6, and gave an NMR spectrum with two signals: delta 6.55 pm and delta 3.84 pm with peak ratio of 2:1. When warmed in pyridine for three hr, compound B quantitatively converts to benzene. Mild hydrogenation of B yielded another compound C with mass spectrum of m/z 82. Infrared spectrum showed no double bonds; NMR spectrum showed one broad peak at delta 2.34 ppm. With this information, address the following questions. a) How many rings are in compound C? b) How many rings are probably in B? How many double bonds are in B? c) Can you suggest a structure for compounds B and C? d) In the NMR spectrum of B, the up-field signal was a quintet, and the down field signal was a triplet. How must you account for these splitting patterns?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
