
a)
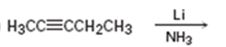
Interpretation:
The product formed and the electron pushing mechanism for its formation, when 2-pentyne is reduced with Li in NH3 is to be given.
Concept introduction:
Lithium metal donates an electron to the
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when 2-pentyne is reduced with Li in NH3.
b)
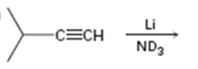
Interpretation:
The product formed and the electron pushing mechanism for its formation, when 3-methyl-1-butyne is reduced with Li in ND3 is to be given.
Concept introduction:
Lithium metal donates an electron to the alkyne to give an anion radical which in the next step abstracts a proton from deuteratedammonia solvent to yield a vinylic radical. In the third step the vinylic radical accepts a second electron from another Li atom to produce a vinylic anion which in the fourth step abstracts another proton from the solvent to yield the final trans product.
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when 3-methyl-1-butyne is reduced with Li in ND3.
c)
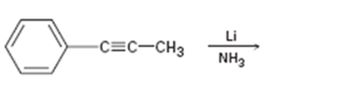
Interpretation:
The product formed and the electron pushing mechanism for its formation, when propynylbenzene is reduced with Li in NH3 is to be given.
Concept introduction:
Lithium metal donates an electron to the alkyne to give an anion radical which in the next step abstracts a proton from ammonia solvent to yield a vinylic radical. In the third step the vinylic radical accepts a second electron from another Li atom to produce a vinylic anion which in the fourth step abstracts another proton from ammonia solvent to yield the final trans product.
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when propynylbenzene is reduced with Li in NH3.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- predict its mechanism and show the detailed mechanism of these reactions using arrows.arrow_forwardPredict the product of the following reaction, and provide the reaction mechanismarrow_forwardSuggest a detailed mechanism for each of the reactions below. Explain why the originating products were formed.arrow_forward
- Show the mechanism and give the expected products for the following reaction.arrow_forwardPredict the major product for the following reaction and give the complete arrow pushing mechanism to show the formation of the product.arrow_forwardPlease show step by step the mechanism and electron flow of the following reaction. thanksarrow_forward
- Provide the mechanism for the following reactions.arrow_forwardUpon oznolysis (oxidative work up), predict the products of the following reagents:arrow_forwardProvide a detailed mechanism for the following (Hint:halohydrin reaction). Use arrows to show electron flow and show intermediates.arrow_forward
- I need help drawing detailed mechanisms for the following reactions.arrow_forwardthanks. Give the full mechanism for the reaction below that leads to the formation of the major monochlorinated product. Include the formation of the electrophile and all of the resonance stabilized arenium ion intermediates.arrow_forwardProvide the final product and mechanism for the following reactionsarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


