
Concept explainers
Draw structures corresponding to the following names:
(a) 3, 3-Dimethyl-4-octyne
(b) 3-Ethyl-5-methyl-1, 6, 8-decatriyne
(c) 2, 2, 5, 5-Tetramethyl-3-hexyne
(d) 3, 4-Dimethylcyclodecyne
(e) 3, 5-Heptadien-1-yne
(f) 3-Chloro-4, 4-dimethyl-1-nonen-6-yne
(g) 3-sec-Butyl-1-heptyne
(h) 5-tert-Buty1-2-methyl-3-octyne
a) 3,3-Dimethyl-4-octyne.
Interpretation:
The structure of 3,3-Dimethyl-4-octyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3,3-dimethyl-4-octyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3,3-dimethyl-4-octyne is
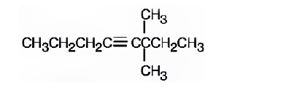
Explanation of Solution
The name shows that the longest carbon chain should contain eight carbons with a triple bond between C4 & C5 and two methyl groups should be present on C3.
The structure corresponding to the IUPAC name 3,3-dimethyl-4-octyne is

b) 3-Ethyl-5-methyl-1,6,8-decatriyne.
Interpretation:
The structure of 3-ethyl-5-methyl-1,6,8-decatriyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3-ethyl-5-methyl-1,6,8-decatriyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3-ethyl-5-methyl-1,6,8-decatriyne is
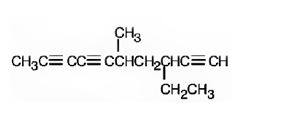
Explanation of Solution
The name shows that the longest carbon chain should contain ten carbons with three triple bonds, one between C1 & C2, another between C6 & C7 a third one between C8 & C9, an ethyl group on C3 and a methyl group on C5.
The structure corresponding to the IUPAC name 3-ethyl-5-methyl-1,6,8-decatriyne is

c) 2,2,5,5- tetramethyl-3-hexyne.
Interpretation:
The structure of 2,2,5,5- tetramethyl-3-hexyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 2,2,5,5- tetramethyl-3-hexyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 2,2,5,5- tetramethyl-3-hexyne is
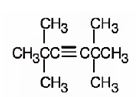
Explanation of Solution
The name shows that the longest carbon chain should contain six carbons with a triple bond between C3 & C4 and four methyl groups, two on C2 and another two on C5.
The structure corresponding to the IUPAC name 2,2,5,5- tetramethyl-3-hexyne is

d) 3,4-Dimethylcyclodeceyne.
Interpretation:
The structure of 3,4-Dimethylcyclodeceyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3,4-dimethyl cyclodeceyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3,4-dimethyl cyclodeceyne is
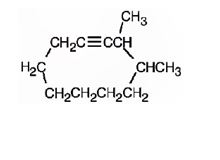
Explanation of Solution
The name shows that the compound contains a ring made up of ten carbon atoms with a triple bond attached to two methyl groups on C3 and C4.
The structure corresponding to the IUPAC name 3,4-dimethyl cyclodeceyne is

e) 3,5-Heptadiene-1-yne.
Interpretation:
The structure of 3,5-heptadiene-1-yne is to be drawn.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. Compounds containing both double bond and triple bonds are called as enynes. The chain is numbered from the end nearer to the multiple bonds, double or triple. When there is a choice in numbering the double bond is given preference and the lowest number is assigned to it.
To draw:
The structure corresponding to the IUPAC name 3,5-heptadiene-1-yne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3,5-heptadiene-1-yne is
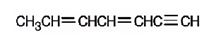
Explanation of Solution
The name shows that the longest carbon chain should contain seven carbons with a triple bond between C1 & C2 and two double bonds, one between C3 & C4 and another between C5 & C6.
The structure corresponding to the IUPAC name 3,5-heptadiene-1-yne is
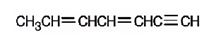
f) 3-Chloro-4,4-dimethyl-1-nonene-6-yne.
Interpretation:
The structure of 3-chloro-4,4-dimethyl-1-nonene-6-yne is to be drawn.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. Compounds containing both double bond and triple bonds are called as enynes. The chain is numbered from the end nearer to the multiple bonds, double or triple. When there is a choice in numbering the double bond is given preference and the lowest number is assigned to it.
To draw:
The structure corresponding to the IUPAC name 3-chloro-4,4-dimethyl-1-nonene-6-yne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3-chloro-4,4-dimethyl-1-nonene-6-yne is
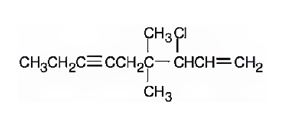
Explanation of Solution
The name shows that the longest carbon chain should contain nine carbons with a triple bond between C6 & C7, one double bond between C1 & C2, a chlorine atom on C3 and two methyl groups on C4.
The structure corresponding to the IUPAC name 3-chloro-4,4-dimethyl-1-nonene-6-yne is

g) 3-sec-Butyl-1-heptyne.
Interpretation:
The structure of 3-sec-Butyl-1-heptyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 3-sec-butyl-1-heptyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 3-sec-butyl-1-heptyne is
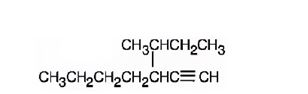
Explanation of Solution
The name shows that the longest carbon chain should contain seven carbons with a triple bond between C1 & C2 and a sec-butyl group on C3.
The structure corresponding to the IUPAC name 3-sec-butyl-1-heptyne is

h) 5-tert-Butyl-2-methyl-3-octyne.
Interpretation:The structure of 5-tert-Butyl-2-methyl-3-octyne is to be drawn.
Concept introduction:
The longest carbon chain containing the triple bond to be chosen. Based on the name of the parent compound–the alkyne name ends with the suffix–yne. The chain is to be numbered from the end that gives the lowest number to the carbon in triple bond. Substituents are to be numbered according to their positions in the chain and listed alphabetically. The position of the triple bond is indicated by giving the number of the first alkyne carbon before the name of the parent name. If more than one triple bond is present, their positions are indicated with the suffixes -diyne, -triyne and so on.
To draw:
The structure corresponding to the IUPAC name 5-tert-Butyl-2-methyl-3-octyne.
Answer to Problem 27AP
The structure corresponding to the IUPAC name 5-tert-Butyl-2-methyl-3-octyne is
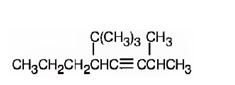
Explanation of Solution
The name shows that the longest carbon chain should contain eight carbons with a triple bond between C3 & C4, a tert-butyl group on C5 and a methyl group on C2.
The structure corresponding to the IUPAC name 5-tert-Butyl-2-methyl-3-octyne is

Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- Two conformations of cis-l, 3-dimethylcyclobutane are shown. What is the difference between them, and which do you think is likely to be more stable?arrow_forwardProvide products for the following reactions. Label them as chiral, achiral, meso, dl, cis/trans, and stereocenters as R,S.arrow_forwardC7H9N has 4 degrees of unsaturation, what are the possible structures?arrow_forward
- Determine the DOU for the following molecules and suggest a structure for each. C5H7Br2ONarrow_forwardDraw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.)E,Z isomerism but not chiralityarrow_forwardDraw the structure(s) of all of the alkene isomers, C5H10, that contain a branched chain. Consider E/Z stereochemistry of alkenes.arrow_forward
- On treatment with Cl2/hν, a compound with the formula C9H12 yields only a single monochloride. What is a possible structure of the compound?arrow_forwardPropose the structure of the following: a. An alkane, C6H14 b. A crylic saturated hydrocarbon, C6H12 c. A diene (dialkene), C5H8 d. A keto alkene, C5H8Oarrow_forwardWhich will be more stable, cis or trans-1,4-tert-butylcyclohexane? Explain by drawing their structures?arrow_forward
- Draw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.) Neither E,Z isomerism nor chiralityarrow_forwardCompound X, C8H17Cl, is a chiral product of the radical chlorination of 4-methylheptane.X reacts in SN2 fashion with NaI in acetone to form Z, C8H17I. When the reactant is the R-enantiomer of X, only the R-enantiomer of Z is formed.Draw a structural formula for X; do not show stereochemistry.arrow_forward3. Which of the following compounds would you expect to have the greatest density: hexane,1-bromohexane, 1-fluorohexane, or 1-chlorohexane? Explain your answer.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

