
Concept explainers
Interpretation:
The structural formulas involved in the sequence of given reactions for the formation of adipic acid or hexamethylene diamine are to be written, with given molecular formula of each compound and IR data of some compound.
Concept introduction:
A
Nylon is a polymer that consists of adipic acid and hexamethylene diamine as monomer units.
Nylon
The formation of Nylon
The addition of hydrogen to double or triple bond is a hydrogenation reaction.
Hydrogenation of
Infrared spectroscopy is a simple, instrumental technique, which helps to determine the presence of various functional groups.
It depends on the interactions of atoms or molecules with the electromagnetic radiation.
The molecules that have dipole moment are IR active and the molecules that do not have dipole moment are IR inactive.
Cyclohexanol shows IR peak at
Cyclic ketone shows IR peak at
Answer to Problem 1PP
Solution:
(a) The structures involved in the sequence are as follows:
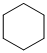 ,
, 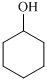 ,
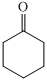
, 
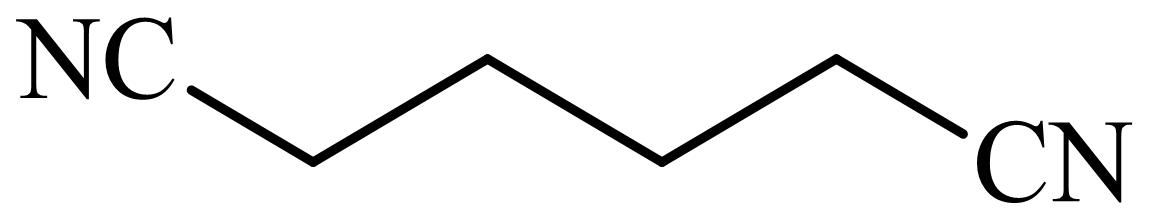
(b) The structures involved in the sequence are as follows:
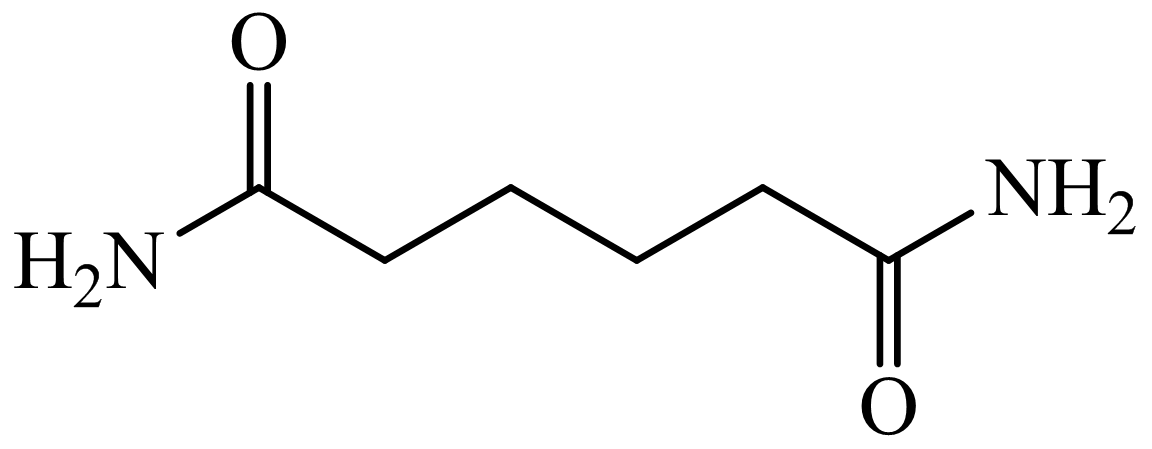 ,
, 
(c) The structures involved in the sequence are as follows:
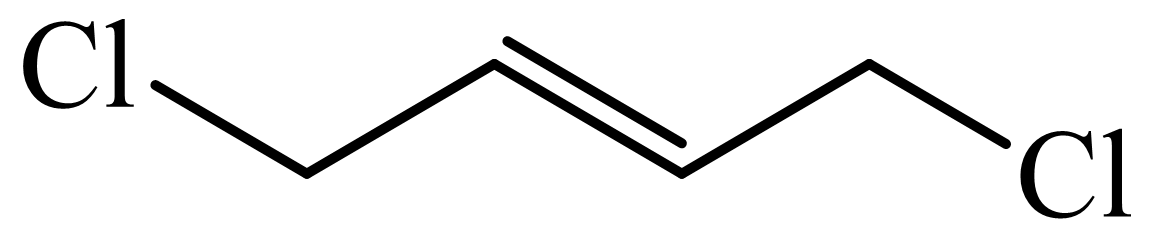 ,
, 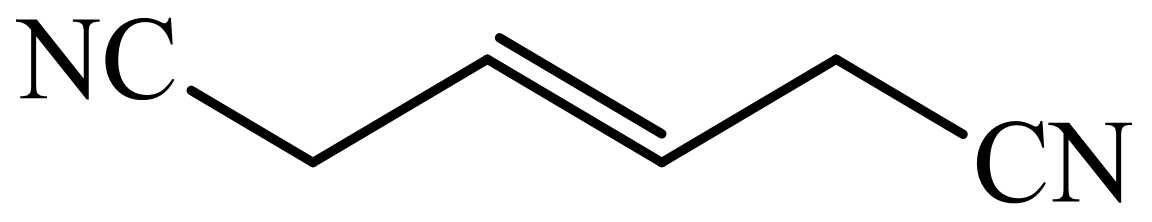 ,
,
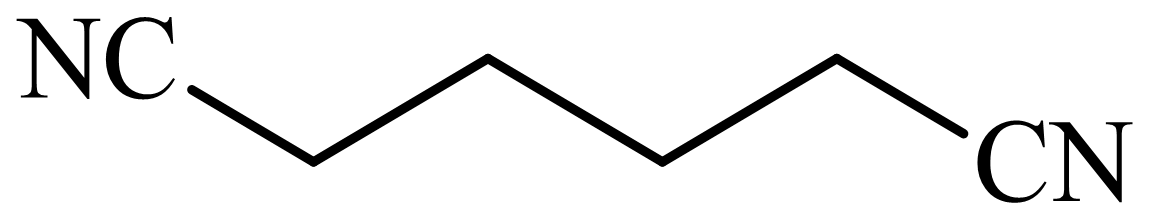
(d) The structures involved in the sequence are as follows:
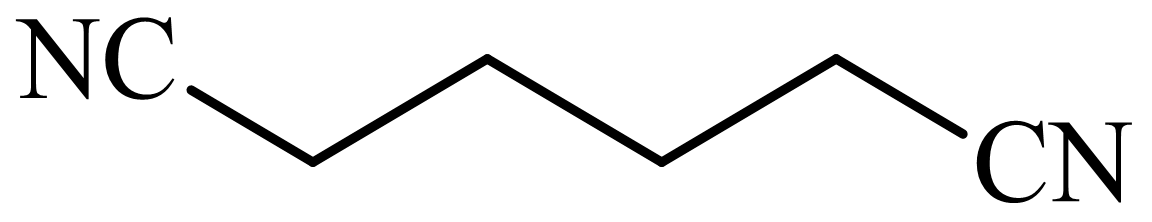
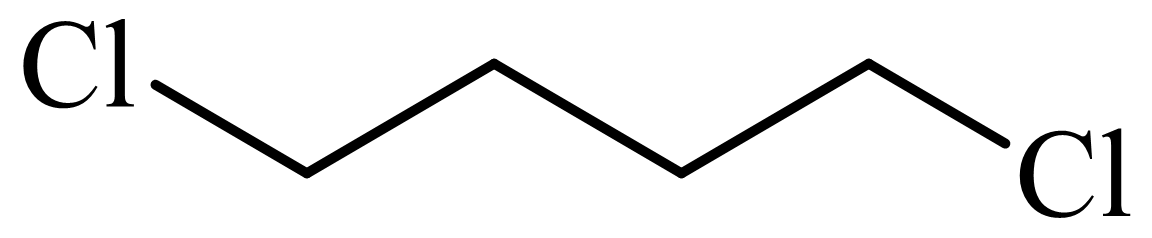 ,
, 
Explanation of Solution
a)
For the formation of adipic acid, first, benzene undergoes hydrogenation reaction to form cyclohexane, which then undergoes oxidation in the presence of catalyst to give cyclohexanol ( I.R. peak at
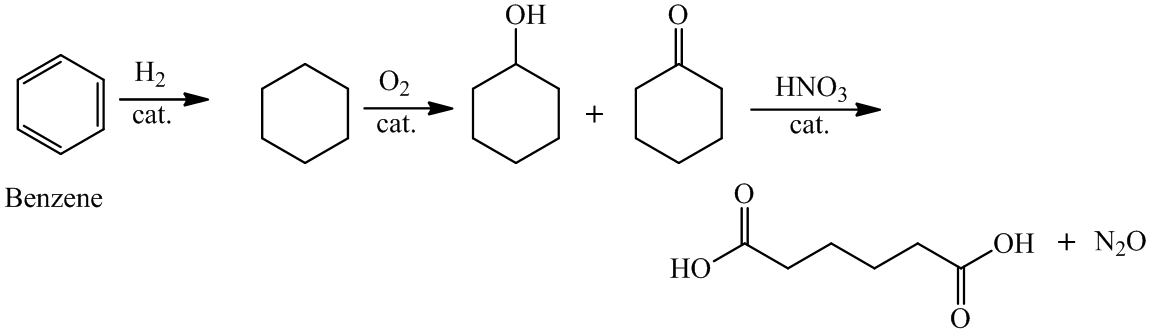
Hence, adipic acid is formed in this sequence of reactions.
b)
Here, adipic acid reacts with two molecules of ammonia to form ammonium salt of adipic acid. Then, on heating, this salt loses two molecules of water to form
The whole sequence can be written as
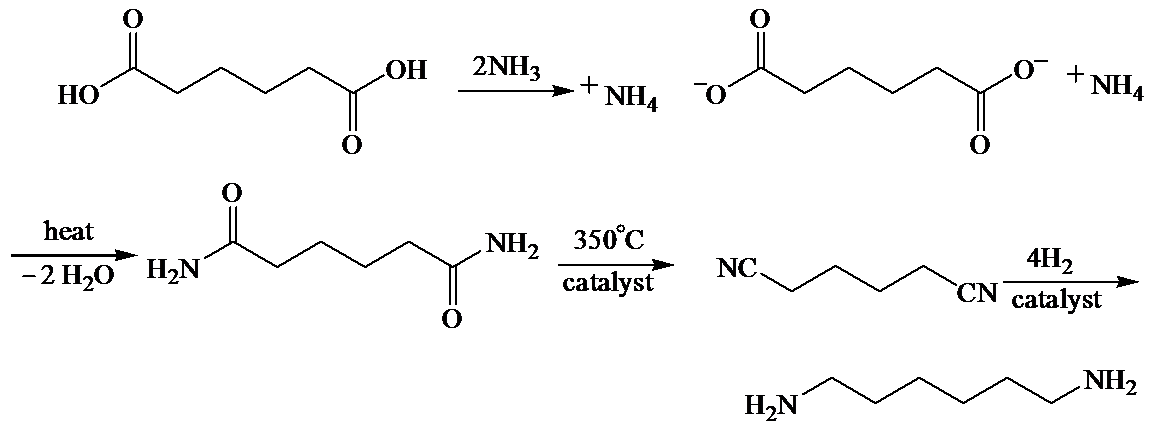
Hence, hexamethylene diamine is formed in this sequence of reactions.
c)
Here,
The whole sequence can be written as
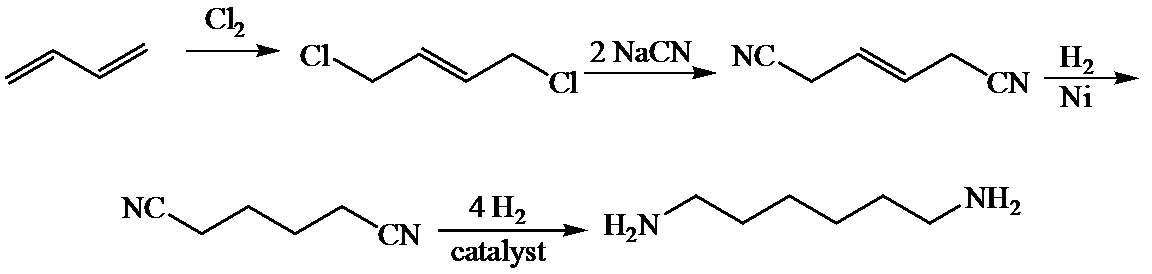
Hence, hexamethylene diamine is formed in this sequence of reactions.
d)
Here, tetrahydrofuran undergoes chlorination in the presence of hydrochloric acid resulting in the formation of
The whole sequence can be written as
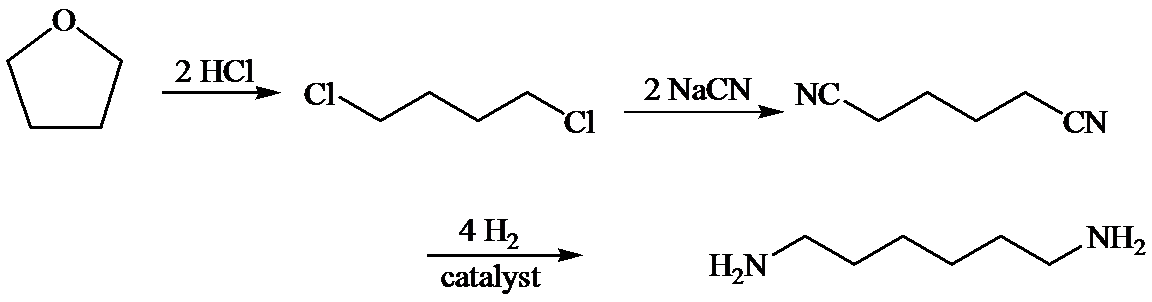
Hence, hexamethylene diamine is formed in this sequence of reactions.
Want to see more full solutions like this?
Chapter E Solutions
Organic Chemistry
- Which of characteristics below describes glycine? Check all that applyarrow_forwardOrganic chemistry exercise. Please provide decent explanation if they are wrong or correct.arrow_forwardcorrect option is 2 I know explaain why it is also I need explaination why other options 1 3 & 4 are incorrect (along with structures) if u won't explaain like this manner I will. downvote for surearrow_forward
- 11-The Nicotine molecule has been found to have a molar mass of 162.1 g / mol and a composition of 74.0% C and 8.70% H and 17.3% N. What is its true formula? Which is the molecular formula?Necessary answer. Unique option. A) C5H7N B) C6H8N2 C) C2H5N D) C10H14N2 E) None of the above.arrow_forwardPlz provide currect answerarrow_forwardPractice question helparrow_forward
- Identify which of the following species have the properties listed below? Select all that apply ?−(???????????)F−(fluorideion) ??−3(??????????)NO3−(nitrateion) ???−3(ℎ???????????????????)HCO3−(hydrogencarbonateion) ?3??4(?ℎ???ℎ????????)H3PO4(phosphoricacid) d) can function as a buffer system componentarrow_forwardWhich of the following alkyl halides are correctly named? Select all that are correct.arrow_forwardSearch on google ptable.com… and do question 6a tell me the property and compound 1 and 2 please do it for me don’t return the question ?arrow_forward
- Which of these structures fit the following descriptions? Select all that are correct.arrow_forwardScenario Four Read the following extract: “A polymer is a substance which has a molecular structure comprising of a large number of identical molecules covalently bonded together to form a long chain structure. Many synthetic organic materials are polymers, such as nylon, polythene and Kevlar.” The extract mentions ‘covalent bonds’. Explain what these are, and the formation of different types that can exist i.e. single, multiple etc. Do some research and identify 4 simply covalently bonded molecules. Draw dot and cross diagrams of each to show the bond formation of each of your 4 examples.arrow_forwardProblem 2-81 onlyarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



