
Concept explainers
Practice Problem F.1
Show how you might use a sulfur ylide to prepare

Interpretation:
The sulfur ylide required for the preparation of the given compounds is to be shown.
Concept introduction:
Sulphur ylide acts as the nucleophile and attacks the carbonyl carbon of aldehydes and ketones.
The intermediate formed in the reaction of sulphur ylide and carbonyl carbon is generally an epoxide rather than an alkene.
Answer to Problem 1PP
Solution:
The reactant used are:
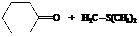
The reactant used are:
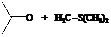
Explanation of Solution
a)
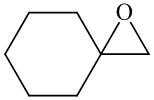
In the formation of the given product, the ketone used is cyclo hexanone. In the first step, sulfur ylide acts as a nucleophile (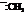 group of sulfur ylide) and attacks the carbonyl carbon of cyclo hexanone. After this,
group of sulfur ylide) and attacks the carbonyl carbon of cyclo hexanone. After this, 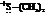 group is removed from the sulfur ylide, which results in the formation of the epoxy ring. The whole reaction can be shown as follows:
group is removed from the sulfur ylide, which results in the formation of the epoxy ring. The whole reaction can be shown as follows:
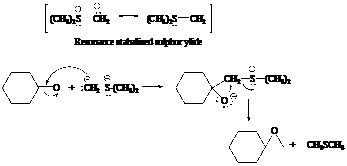
Hence, cyclohexane is used in combination with sulfur ylide to form the given compound.
b)
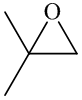
In the formation of the given product, the ketone used is acetone. In the first step, sulfur ylide acts as a nucleophile (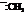 group of sulfur ylide) and attacks the carbonyl carbon of the acetone. After this,
group of sulfur ylide) and attacks the carbonyl carbon of the acetone. After this, 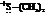 group is removed from the sulfur ylide which results in the formation of the epoxy ring.
group is removed from the sulfur ylide which results in the formation of the epoxy ring.
The whole reaction can be shown as follows:
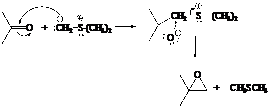
Hence, acetone is used in combination with sulfur ylide to form the given compound.
Want to see more full solutions like this?
Chapter F Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry
Chemistry & Chemical Reactivity
Chemistry: Matter and Change
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Organic Chemistry (8th Edition)
Living By Chemistry: First Edition Textbook
- Organic Chemistry problem Organolithium formation Question Please help. Thank you.arrow_forwardWhich of the following species should not be present in a base-catalyzed mechanism? (select all that apply)arrow_forwardchoose correct option and give detail explanation for correct and incorect answerarrow_forward
- (please show reaekson and incorrect options is explan and correct answer )arrow_forwardA,B,C do not need answeredarrow_forwardThapsigargin is a natural product with promising anticancer properties. Question : At which sites can thapsigargin hydrogen bond to another molecule like itself?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





