
(a)
Interpretation:
Given chemical equation has to be balanced and also the oxidizing agent and reducing agent has to be identified.
Concept Introduction:
In
In redox reactions, reducing agent is the one that gets oxidized by causing reduction. These agents can be ions, elements, or even compounds. In reduction, the oxidation number decreases due to gain of electrons.
(a)
Answer to Problem K.17E
Balanced chemical equation is
Explanation of Solution
The given reaction is written as follows;
The above chemical equation has the same number of atoms of elements equal on both sides. Hence, this itself is a balanced equation.
Oxidation number of the atoms present in the above equation is indicated as follows;
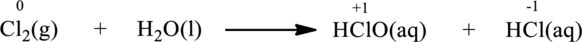
From the above equation, it is found that the oxidation state of chlorine is increased from
The oxidation state of chlorine decreases from
(b)
Interpretation:
Given chemical equation has to be balanced and also the oxidizing agent and reducing agent has to be identified.
Concept Introduction:
Refer part (a).
(b)
Answer to Problem K.17E
Balanced chemical equation is;
Oxidizing agent is
Explanation of Solution
The given reaction is written as follows;
Balancing Hydrogen atoms: In the left side of the equation there are two hydrogen atoms while on the product side only one hydrogen atom is present. Adding coefficient
Balancing Sodium atoms: In the left side of the equation there is one sodium atom while on the product side there are two sodium atoms. Adding coefficient
Balancing Chlorine atoms: In the left side of the equation there are two chlorine atoms while on the product side there is one chlorine atom. Adding coefficient
Oxidation number of the atoms present in the above equation is indicated as follows;
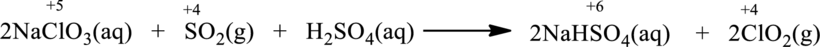
From the above equation, it is found that the oxidation state of sulfur is increased from
The oxidation state of chlorine decreases from
(c)
Interpretation:
Given chemical equation has to be balanced and also the oxidizing agent and reducing agent has to be identified.
Concept Introduction:
Refer part (a).
(c)
Answer to Problem K.17E
Balanced chemical equation is
Explanation of Solution
The given reaction is written as follows;
Balancing iodine atom: In the reactant side, there is one iodine atom while on the product side, there are two iodine atoms. Adding coefficient
Balancing copper atom: In the reactant side, there are two copper atoms while on the product side, there is one copper atom. Adding coefficient
Oxidation number of the atoms present in the above equation is indicated as follows;
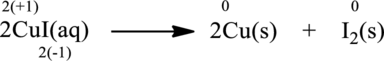
From the above equation, it is found that the oxidation state of iodine is increased from
The oxidation state of copper decreases from
Want to see more full solutions like this?
Chapter F Solutions
Chemical Principles: The Quest for Insight
- One of the few industrial-scale processes that produce organic compounds electrochemically is used by the Monsanto Company to produce1,4-dicyanobutane. The reduction reaction is 2CH2CHCH+2H++2eNC(CH2)4CN The NC(CH2)4CN is then chemically reduced using hydrogen gas to H2N(CH2)6NH2, which is used in the production of nylon. What current must be used to produce 150.kg NC(CH2)4CN per hour?arrow_forward1. Sometimes a reaction can fall in more than one category. Into what category (or categories) does the reaction of Ba(OH)2(aq) + H+PO4(aq) fit? acid-base and oxidation-reduction oxidation-reduction acid-base and precipitation precipitationarrow_forwardThe iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing small water-soluble ions and molecules. The iron in the aqueous solution is reduced to iron(II) ion and then titrated against potassium permanganate. In the titration, iron(ll) is oxidized to iron(III) and permanganate is reduced to manganese(II) ion. A 5.00-g sample of hemoglobin requires 32.3 mL of a 0.002100 M solution of potassium permanganate. The reaction with permanganate ion is MnO4(aq)+8H+(aq)+5Fe2+(aq)Mn2+(aq)+5Fe3+(aq)+4H2O What is the mass percent of iron in hemoglobin?arrow_forward
- An electrode is prepared from liquid mercury in contact with a saturated solution of mercury(I) chloride, Hg2Cl, containing 1.00 M Cl . The cell potential of the voltaic cell constructed by connecting this electrode as the cathode to the standard hydrogen half-cell as the anode is 0.268 V. What is the solubility product of mercury(I) chloride?arrow_forwardTriiodide ions are generated in solution by the following (unbalanced) reaction in acidic solution: IO3(aq) + I(aq) I3(aq) Triiodide ion concentration is determined by titration with a sodium thiosulfate (Na2S2O3) solution. The products are iodide ion and tetrathionate ion (S4O6). a. Balance the equation for the reaction of IO3 with I ions. b. A sample of 0.6013 g of potassium iodate was dissolved in water. Hydrochloric acid and solid potassium iodide were then added. What is the minimum mass of solid KI and the minimum volume of 3.00 M HQ required to convert all of the IO3 ions to I ions? c. Write and balance the equation for the reaction of S2O32 with I3 in acidic solution. d. A 25.00-mL sample of a 0.0100 M solution of KIO. is reacted with an excess of KI. It requires 32.04 mL of Na2S2O3 solution to titrate the I3 ions present. What is the molarity of the Na2S2O3 solution? e. How would you prepare 500.0 mL of the KIO3 solution in part d using solid KIO3?arrow_forwardThe carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward
- Aluminum is produced commercially by the electrolysis of Al2O3 in the presence of a molten salt. If a plant has a continuous capacity of 1.00 million A, what mass of aluminum can be produced in 2.00 h?arrow_forwardThe Ostwald process for the commercial production of nitric acid involves the Following three steps: 4NH3(g)+5O2(g)4NO(g)+6H2O(s)2NO(g)+O2(g)2NO2(g)3NO2(g)+H2O(l)2HNO3(aq)+NO(g) a. Which reaction in the Ostwald process are oxidation-reduction reactions? b. Identify each oxidizing agent and reducing agent.arrow_forwardMagnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions: Mg2+(aq)+Ca(OH)2(aq)Mg(OH)2(s)+Ca2+(aq)Mg(OH)2(s)+2HCl(aq)MgCl2(s)+2H2O(l)MgCl2(l)electrolysisMg(s)+Cl2+Cl2(g) Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium a5 Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2; is required to precipitate 99.9% of the magnesium in 1.00103 L of sea water?arrow_forward
- Bromine is obtained from sea water by the following redox reaction: Cl2(g) + 2 NaBr(aq) 2 NaCl(aq) + Br2() (a) What has been oxidized? What has been reduced? (b) Identify the oxidizing and reducing agents.arrow_forwardOrder the following oxidizing agents by increasing strength under standard-state conditions: Mg2+(aq), Hg2+(aq), Pb2+(aq).arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





