
Concept explainers
Practice Problem G.1
When farnesol is treated with sulfuric acid, it is converted to bisabolene. Outline a possible mechanism for this reaction.
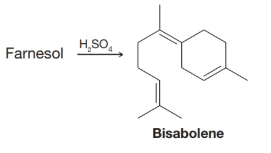
Interpretation:
The mechanism for the formation of Bisabolene from Farnesol in presence of 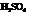 is to be outlined.
is to be outlined.
Concept introduction:
Farnesol is a compound that is formed from isoprene compounds. In general, when geranyl pyrophosphate reacts with isopenenyl pyrophosphate, farnesyl pyrophosphate (intermediate for the synthesis of sesquiterpenes) is formed. This, on oxidation can give farnesol.
Concentrated sulfuric acid acts as a strong dehydrating agent and removes the water molecule from alcohols to form unsaturated hydrocarbon.
Answer to Problem 1PP
Solution:
The mechanism for the synthesis of Bisabolene is explained as follows:

Explanation of Solution
During the first step, Farnesol (unsaturated alcohol) reacts with acid (HA = 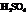 ) to form a hydrated product. Then, the hydrated product formed loses one water molecule to form a carbocation. Consequently, this carbocation rearranges to form a more stable carbocation. At last, the base
) to form a hydrated product. Then, the hydrated product formed loses one water molecule to form a carbocation. Consequently, this carbocation rearranges to form a more stable carbocation. At last, the base 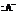 (
( 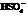 ) abstracts the hydrogen ion from the molecule, forming Bisabolene.
) abstracts the hydrogen ion from the molecule, forming Bisabolene.
The whole mechanism can be depicted as:

Hence, Bisabolene has been formed from Farnesol in the presence of concentrated sulfuric acid.
The Bisabolene has been synthesized from Farnesol in the presence of concentrated sulfuric acid. The mechanism for the synthesis of Bisabolene is outlined as:

Want to see more full solutions like this?
Chapter G Solutions
Organic Chemistry
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
CHEMISTRY-TEXT
Chemistry: Structure and Properties (2nd Edition)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Chemistry: A Molecular Approach
- Which of the following reaction sequences will produce the product below? Select ALL that apply.arrow_forwardWhich of the following species should not be present in a base-catalyzed mechanism? (select all that apply)arrow_forward(course name: organic synthesis technique) question:Develop a procedure for the synthesis of any molecule and draw the mechanism figures. I hope the answer is written by computerarrow_forward
- are the reactants correct to produce N-Ethyl-2-phenylethanamine and show mechanismarrow_forwardThapsigargin is a natural product with promising anticancer properties. Question : At which sites can thapsigargin hydrogen bond to another molecule like itself?arrow_forwardHello, I do not understand these questions and I am stuck. May I get help please?? Question: draw the MAJOR organic product(s) for the following reactions. If no reaction occurs state no reaction in the box providedarrow_forward
- Show mechanism of boron trichloride (trichloroborane) with boron tribromide (tribromoborane) to give a mixture of products. What are the molecular formulas of product species?arrow_forwardpredict products no mechanismarrow_forwardQuestion: Turn the starting mass of acetanilide to moles, show the calculation with units for full credit. Given starting mass was 1.0 g of acetanilide into a 100 mL beaker. Used 2 g of N bromo succinimde, and 0.230 g of mandelic acid. ( Bromination of acetanilide:electrophilic substitution reaction) Please solve and explain B. Assuming that the acetanilide is the limiting reagent, calculate the theoretical yield of bromo-acetanilide product.arrow_forward
- identify the reagents a-b in the following scheme: Please provide only typed answer solution no handwritten solution needed allowedarrow_forwardWhich of the following diols ARE likely to undergo oxidative change with H2CrO4? Select all that applyarrow_forwardBoth question...product & mechanism...arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





