
Concept explainers
Interpretation:
The geometry around the nitrogen and boron centers in the addition compound,
Concept Introduction:
VSEPR or valence shell electron pair repulsion theory predicts the shape of the molecules with the use of arrangement of electrons around the central metal atom. Its postulates are as follows:
1. The shape of the molecules is governed by the repulsion between electron pairs situated in the outermost or valence shell of the atoms.
2. A distortion is observed in the shape of the molecules if lone pairs are present in it. These are the electron pairs that do not participate in the
3. The order of repulsion of electron pairs is as follows:
4. Multiple bonds create more repulsion as compared to single bonds.
5. The following table shows the various molecular shapes that match to different number of electron pairs:
The geometry of the molecule is predicted with the help of steric number. The formula to calculate the steric number is as follows:
The least electronegative atom is usually taken to be the central atom.
Answer to Problem 1PQ
Option (A) is the correct one.
Explanation of Solution
Reason for correct option:
The formation of
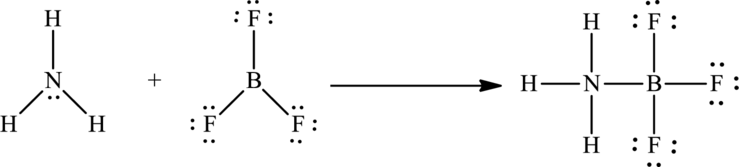
Substitute 4 for atoms bonded to the central atom and 1 for number of lone pairs on the central atom in equation (1) to calculate the steric number of
So the number of electron pairs around nitrogen atom is 4 and no lone pairs are present on it. Therefore the geometry around this atom is tetrahedral according to the VSEPR theory.
Substitute 4 for atoms bonded to the central atom and 1 for number of lone pairs on the central atom in equation (1) to calculate the steric number of
So the number of electron pairs around boron atom is 4 and no lone pairs are present on it. Therefore the geometry around this atom is also tetrahedral according to the VSEPR theory.
Therefore the geometry around both the nitrogen and boron atoms in the addition compound
Reason for incorrect option:
Since geometry around both
The geometry around the nitrogen and boron centers in the addition compound
Want to see more full solutions like this?
Chapter MS Solutions
Preparing for your ACS examination in general chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





