1) The Ideal Gas Law, PV = RT, relates the pressure (P, in pascals), temperature (7, in Kelvin), and volume (V, in cubic meters) of 1 mole of a gas (with R = 8.314 as the universal gas constant), and describes the behavior of gases that do not liquefy easily, such as oxygen and hydrogen. We can solve the ideal gas law for volume and hence treat the volume as a function of the pressure and mol K temperature: V(P,T) = 8.314T P a) Explain in detail what the trace of V with P = 1000 tells us about a key relationship between two quantities. b) Explain in detail what the trace of V with T = 5 tells us. c) Explain in detail what the level curve V = 0.5 tells us. d) Use 2 or 3 additional traces in each direction to make a rough sketch of the surface over the domain of V where P and T are non-negative. Write at least one sentence that describes the way the surface looks. e) Based on all your work above, write a couple of sentences that describe the effect that temperature and pressure have on volume.
1) The Ideal Gas Law, PV = RT, relates the pressure (P, in pascals), temperature (7, in Kelvin), and volume (V, in cubic meters) of 1 mole of a gas (with R = 8.314 as the universal gas constant), and describes the behavior of gases that do not liquefy easily, such as oxygen and hydrogen. We can solve the ideal gas law for volume and hence treat the volume as a function of the pressure and mol K temperature: V(P,T) = 8.314T P a) Explain in detail what the trace of V with P = 1000 tells us about a key relationship between two quantities. b) Explain in detail what the trace of V with T = 5 tells us. c) Explain in detail what the level curve V = 0.5 tells us. d) Use 2 or 3 additional traces in each direction to make a rough sketch of the surface over the domain of V where P and T are non-negative. Write at least one sentence that describes the way the surface looks. e) Based on all your work above, write a couple of sentences that describe the effect that temperature and pressure have on volume.
Related questions
Question
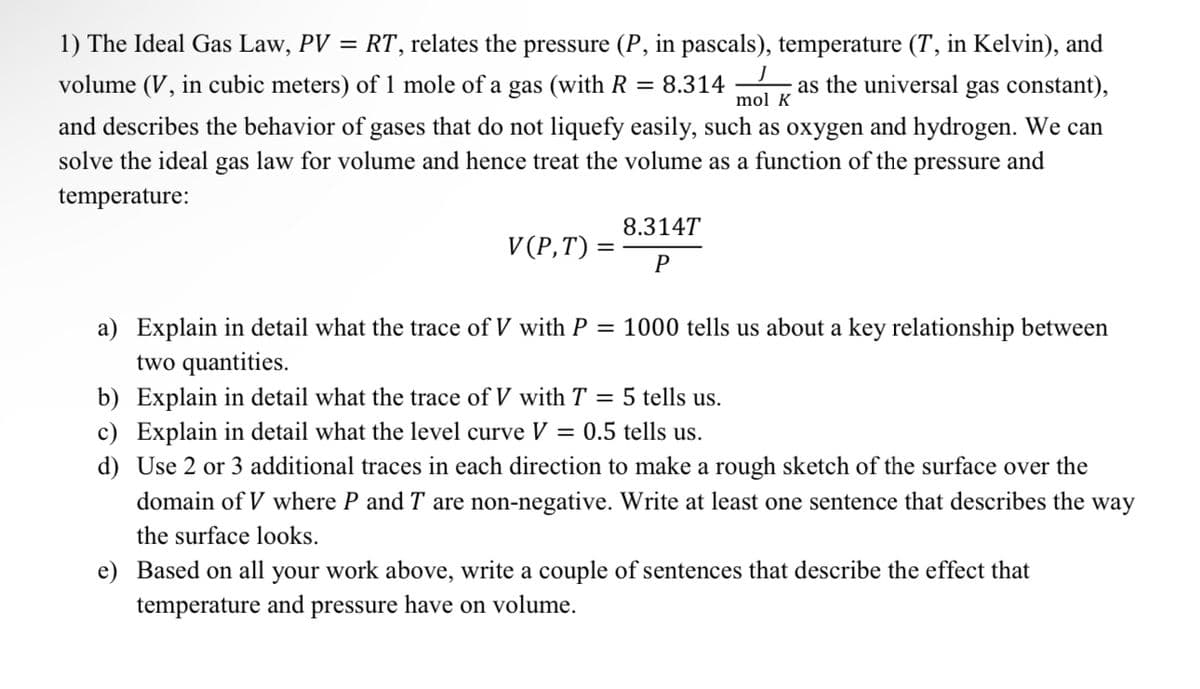
Transcribed Image Text:mol K
1) The Ideal Gas Law, PV = RT, relates the pressure (P, in pascals), temperature (T, in Kelvin), and
volume (V, in cubic meters) of 1 mole of a gas (with R = 8.314 as the universal gas constant),
and describes the behavior of gases that do not liquefy easily, such as oxygen and hydrogen. We can
solve the ideal gas law for volume and hence treat the volume as a function of the pressure and
temperature:
V(P,T) =
8.314T
P
1000 tells us about a key relationship between
a) Explain in detail what the trace of V with P
two quantities.
b) Explain in detail what the trace of V with T = 5 tells us.
c) Explain in detail what the level curve V = 0.5 tells us.
d) Use 2 or 3 additional traces in each direction to make a rough sketch of the surface over the
domain of V where P and T are non-negative. Write at least one sentence that describes the way
the surface looks.
e) Based on all your work above, write a couple of sentences that describe the effect that
temperature and pressure have on volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 7 images
