1. Which of the following catalyst best describes when the catalyst and the reactant(s) are in different phases, with the reaction occurring at the interface between them (most commonly, the gas-solid "border")? A. Enzymes C. Heterogeneous Catalyst B. Catalytic Poison D. Homogeneous Catalyst
1. Which of the following catalyst best describes when the catalyst and the reactant(s) are in different phases, with the reaction occurring at the interface between them (most commonly, the gas-solid "border")? A. Enzymes C. Heterogeneous Catalyst B. Catalytic Poison D. Homogeneous Catalyst
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.11PAE: Identify each of the processes listed as spontaneous or non-spontaneous. For each non spontaneous...
Related questions
Question
Please help me answer these subunits!
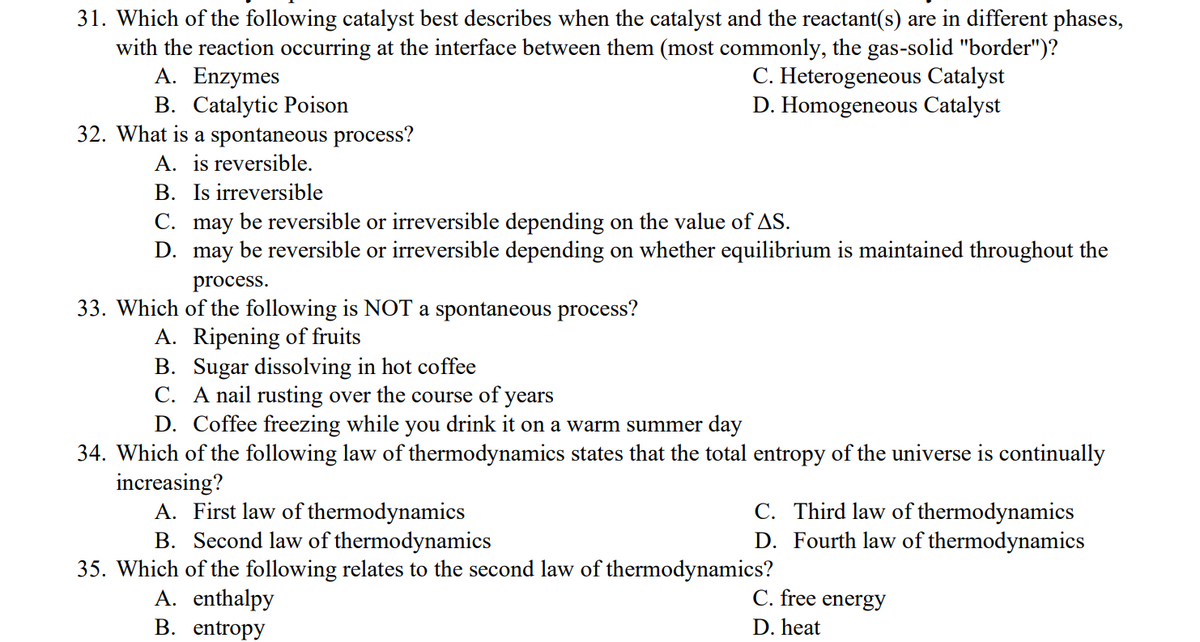
Transcribed Image Text:31. Which of the following catalyst best describes when the catalyst and the reactant(s) are in different phases,
with the reaction occurring at the interface between them (most commonly, the gas-solid "border")?
A. Enzymes
C. Heterogeneous Catalyst
B. Catalytic Poison
D. Homogeneous Catalyst
32. What is a spontaneous process?
A. is reversible.
B. Is irreversible
C. may be reversible or irreversible depending on the value of AS.
D.
may be reversible or irreversible depending on whether equilibrium is maintained throughout the
process.
33. Which of the following is NOT a spontaneous process?
A. Ripening of fruits
B. Sugar dissolving in hot coffee
C. A nail rusting over the course of years
D. Coffee freezing while you drink it on a warm summer day
34. Which of the following law of thermodynamics states that the total entropy of the universe is continually
increasing?
A. First law of thermodynamics
C. Third law of thermodynamics
D. Fourth law of thermodynamics
B. Second law of thermodynamics
35. Which of the following relates to the second law of thermodynamics?
A. enthalpy
C. free energy
B. entropy
D. heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning