1. Which of the following is NOT a characteristic of liquids? A. Liquids have the ability to flow. B. Liquids conform to the shape of their container. C. The particles of a liquid are not attracted to each other. D. The particles of liquids are closer together than particles of gases. 2. When NaCl dissolves in water, aqueous Na+ and Cl ions result. What do you call the force of attraction that exists between Na+ and H₂O? A. dipole-dipole C. ion-dipole B. hydrogen bonding D. London dispersion force 3. Which statement is true about the viscosity of a liquid? A. The smaller the molecules, the more viscous the liquid. B. The higher the temperature, the more viscous the liquid. C. The stronger the intermolecular forces, the more viscous the liquid. D. The intermolecular forces of the liquid are not related to its viscosity.
1. Which of the following is NOT a characteristic of liquids? A. Liquids have the ability to flow. B. Liquids conform to the shape of their container. C. The particles of a liquid are not attracted to each other. D. The particles of liquids are closer together than particles of gases. 2. When NaCl dissolves in water, aqueous Na+ and Cl ions result. What do you call the force of attraction that exists between Na+ and H₂O? A. dipole-dipole C. ion-dipole B. hydrogen bonding D. London dispersion force 3. Which statement is true about the viscosity of a liquid? A. The smaller the molecules, the more viscous the liquid. B. The higher the temperature, the more viscous the liquid. C. The stronger the intermolecular forces, the more viscous the liquid. D. The intermolecular forces of the liquid are not related to its viscosity.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.25QP: Consider two hypothetical pure substances, AB(s) and XY(s). When equal molar amounts of these...
Related questions
Question
Please help me answer these subunits!
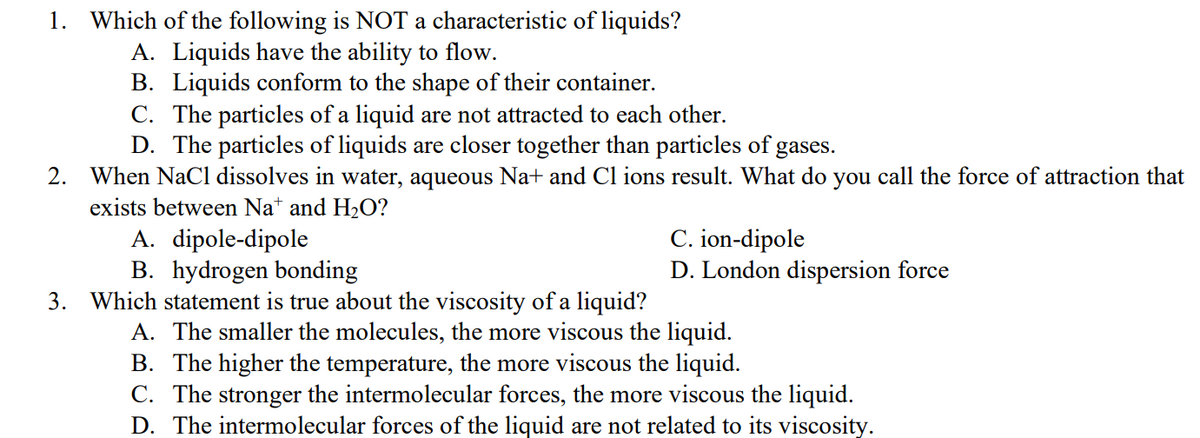
Transcribed Image Text:1. Which of the following is NOT a characteristic of liquids?
A. Liquids have the ability to flow.
B. Liquids conform to the shape of their container.
C. The particles of a liquid are not attracted to each other.
D. The particles of liquids are closer together than particles of gases.
2. When NaCl dissolves in water, aqueous Na+ and Cl ions result. What do you call the force of attraction that
exists between Na+ and H₂O?
A. dipole-dipole
C. ion-dipole
B. hydrogen bonding
D. London dispersion force
3. Which statement is true about the viscosity of a liquid?
A. The smaller the molecules, the more viscous the liquid.
B. The higher the temperature, the more viscous the liquid.
C. The stronger the intermolecular forces, the more viscous the liquid.
D. The intermolecular forces of the liquid are not related to its viscosity.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
