
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
PROBLEM 7) Can you explain this problem and solve it correctly, please!
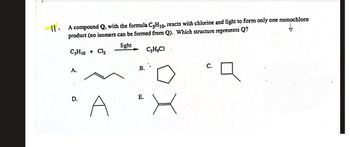
Transcribed Image Text:11. A compound Q, with the formula C5H10, reacts with chlorine and light to form only one monochloro
product (no isomers can be formed from Q). Which structure represents Q?
C5H10 + Cl₂
light
C₂H₂CI
A.
D.
B.
E.
A
C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Complete and balance the following combustion reactions. Assume that each hydrocarbon is converted completely to carbon dioxide and water. (a) Propane + O2 (b) Octane + O2 (c) Cyclohexane + O2 (d) 2-Methylpentane + O2arrow_forward3. Like polymers, dendrimers are macromolecules (large molecules). Some dendrimers have been used in applications like drug delivery and waste water reclamation. What is the result of the following reaction producing a "first generation" dendrimer? NH2 H2N. (-HCI) ???? 4 NH2arrow_forward8. Three compounds, G, H and J are structural isomers. They have the molecular formula C3H$O. Their chemical properties are given below: React with 2,4-dinitrophenylhydrazine to form yellow precipitate. G is resistant to oxidation. H is oxidised to L (C7H6O2) J is oxidised to M (C8H6O4) Write the structural formula of G, H, J, L and M. Outline one chemical test to distinguish between G and H.arrow_forward
- B. Θ (CH3)3NH CIO D. H3CC=CCH₂OHarrow_forward3. Balance the following equation for the reaction of ethyl alcohol with oxygen: olsesup O2(g) C2H6O(g) CO2(g) H20(1) + als: sle eme bns DH ME alsharrow_forwardWhich of the organic compounds shown below exhibit cis-trans isomerism? Select one: a. CH3CH=CHCH2CH3 O b. CH2=CH(CH3) O c. CH2=C(CH3)2 O d. CH2=C(CI)2arrow_forward
- (Picture attached) Functional Groups: 1. Benzene 2. Halogen 3. Carboxyl 4. Hydroxyl Identify the functional groups that the 2 molecules contain. Note: each functional group can be used more than once. Put in numerical order with no space. Sucarlose = Ibuprofen =arrow_forwardDetermine the functional groups of the followingarrow_forwardSnO2 + H2 ? Sn + H2O 'arrow_forward
- draw the structure and give the systematic name of a compound with molecular formula C5H12 that a. only primary and secondary halogens b. only primary halogens c. one tertiary hydrogensarrow_forwardодн 2. H. OH NH₂ H a mixture of two isomers + Et3N + H₂O (C8H14) NaBH3CN (C8H15N)arrow_forwardFf.224.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning