
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
PROBLEM 19) Can you explain this problem and solve it correctly, please!
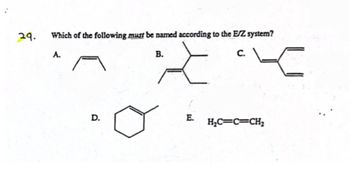
Transcribed Image Text:29.
Which of the following must be named according to the E/Z system?
A.
B.
C.
D.
E.
H₂C=C=CH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- 1 a. b. C. d. e. f. g. CH3OH C₂H5OH H3C CH3 H3C ਸ HBr 1. NaH CH3 2. C₂H5CI Br H3C -H H- HO H CH3 H CH3 CH₂CH3 H3CH₂C H3C H 27 H Br -CH₂CH₂CH3 CH₂CH₂CH3 H3CH₂C CH3OH tº CH3ONa tº H OH H CH₂CH₂CH3 H3C NaOC₂H5 PBr3 CH3O OH SOCI₂ CH3 CH₂CH₂CH3 HBr CH3 нотн CH₂CH₂CH3 NaH DMSO CH3ONa tºarrow_forwardWhich of the molecules gives rise to a molecular ion with an odd value of m/z? A. Br2C8H14 B. NC8H13 C. Cl2C6H12 D. N2C7H10arrow_forwardHi! These questions are answerable by NON-POLAR or POLAR.arrow_forward
- 3. a. E,R is? b. E,S c. S,Z 4. What is the π-energy diagram of the compound in question 3? a. b. d. R,Z e. not a.-d. d. e. not a.-d.arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 nex to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species ? H3PO4 3 НСОО |(Choose one) HC,04 |(Choose one) 2. H,C,04 2 + H;O* H,0 |(Choose one) НСООН 4arrow_forward6:01 | LTE O ( To Do Assignment Details 03101 Chemistry I 2122-FYa - Sem 2 Which atom is the main #3 "Oxygen" component of organic (Carbabo) molecules? "Cyclohexene is a linear molecule Describe the with 6 carbon molecule atoms and 12 hydrogen atoms. There is a triple bond because it #4 cyclohexene and provide the (Glycerina) chemical ends in -ene. The formula. chemical formula is C6H12" Which two functional groups are in the molecule "The left shown functional group is a carboxylic acid. The right functional group is an alcohol." #5 below? Use (Trinitress) the terms left and right to distinguish them. in Discussion Responses Additionally, reply to at least one classmate with View Discussion Dashboard Calendar To Do Notifications Inbox 因arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

