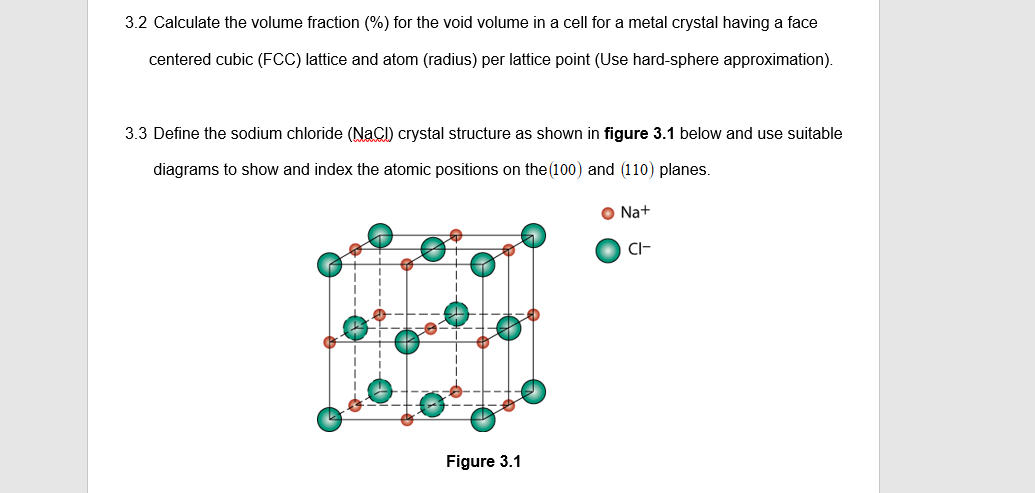
3.2 Calculate the volume fraction (%) for the void volume in a cell for a metal crystal having a face centered cubic (FCC) lattice and atom (radius) per lattice point (Use hard-sphere approximation).
Q: Why do astronomers refer to carbonaceous chondrites as unmodified or "primitive" material?
A: Chondrites or stony meteorites are the most common types of meteorites in a meteorite fall. Roughly…
Q: 5m of ecadius 1 mm is A mive of length stretched by load of 10 N. If Y-2x10" N/m² find work done peu…
A: We need to find-Work done per unit volume (W)=?Given-Length of wire (L)=5 mRadius of wire (r)=1…
Q: When two identical resistors are wired in series, the equivalent resistance is 2.5 ohms. What is the…
A: Given that-Equivalent resistance, R= 2.5 ΩVoltage applied across the resistors, V= 9 VoltWe are…
Q: The constant tensions of 200 N and 160 N are ap- plied to the hoisting cable as shown. If the…
A: The tension transmitted by a cable, rope, wire, or string occurs when forces acting on opposite ends…
Q: How does the dynamo effect take place in Neptune?
A: The Dynamo effect is the generation of a magnetic field due to rotation of the core in a planet.…
Q: 8) Star A has an apparent magnitude of 2.5 and star B has an apparent magnitude of 3.9. Calculate…
A: When studying stars astronomers use two different magnitudes, apparent and absolute magnitude.…
Q: 6 10 2 2 t (ms) 6 The current i through a 4.1 H inductor varies with time t as shown by the graph of…
A: Given Inductance L = 4.1 H Resistance R = 12 Ω Find the magnitude in volts of the induced emf &…
Q: Given that Earth is about 4.6 billion (4.6 × 10%) years old, how many precessional cycles have…
A: Precession takes place when the elliptical orbit path of an object rotates around its center.…
Q: The equipotential lines in a region of electric field are shown in the what is the work done by the…
A: Work done in moving a charge from one point to another point is opposed by the change in electric…
Q: In physics, what is the finest use of inferential calculus?
A: Required : The finest use of inferential calculus in physics.
Q: How does the dynamo effect account for the magnetic fields of Jupiter?
A: Jupiter is a gas giant and falls under the category of Jovian planets. Jovian planets are those…
Q: A current of 5 A flows through an electric heater of resistance 22 Q. The voltage drop across the…
A: Given that-Current in heater, I= 5AResistance of heater, R= 22ΩThe voltage drop across the heater,…
Q: Where does the brain of the electrical system reside?
A: introduction: The majority of people don't appear to view the brain as electric; rather, they…
Q: Why are Uranus and Neptune respectively green-blue and blue?
A: Uranus and Neptune are ice giants. They are similar in size and have a deep atmosphere. Their…
Q: "ind the geometrica ical structu ollowing reflection (100), C n the diffraction pattern?
A: Given: Consider the given pattern of sequence number of reflection (100), (110), (220), (222),…
Q: How do you explain the concept of particle-wave duality?
A:
Q: = [₁₂ (a 2 x(x + 1) 8 (x³ - x) dx
A:
Q: a. Write down the energy eigenfunctions for a particle in an infinitely deep one- dimensional square…
A:
Q: A current-carrying conductor passes through a square region of magnetic field, magnitude 0.5 T, as…
A:
Q: A 50 g silver disk at 3000C is placed in 200 cm3 of methyl alcohol at 140C, then removed quickly.…
A: We can say-When the temperature of the hot silver disc dropped, the disc lost heat, which was…
Q: Why do astronomers think the asteroids were never part of a full-sized planet?
A: Asteroids are rocky objects that orbit Sun. They are very small to be considered a planet. They are…
Q: Only first part
A: Given that-Potential difference, V= 71 VoltCross-sectional area= 3.56×10-6 m2Resistivity, ρ=…
Q: What is meant by electromagnetism? Choose which two applications of electromagnetism are used in…
A: Electromagnetism is a branch of physics that studies how magnetic fields interact with matter and…
Q: In a region, potential is a function of x only, i.e. potential does not change in the y- or…
A:
Q: Prove: =0 = √√ntl H n ħ 2mw <n-1|2|m²) = √²m² ₁ + 0 n
A:
Q: a second pendulum due to gequity changes from Find change in length of it acceleration 9.65m/s² to…
A: We need to find-Change in length l2-l1=?Given that-g=9.65 m/s2g'=9.8 m/s2Time period of seconds…
Q: In physics, what is the finest use of inferential calculus?
A: A subset of statistics called inferential statistics uses a variety of analytical techniques to…
Q: (a) the number of binary links, (b) the number of ternary links, (c) the number of total links, (d)…
A: Solution is explained below in details .. please rate me helpful if you like my efforts
Q: Venus-orbiting geosynchronous satellite. Venus rotates every 117 days. How far above Venus's surface…
A: Given, Venus orbiting geosynchronous satellite. and time period of rotation is 117 days.
Q: Standard automobile batteries have six lead-acid cells in series, creating a total emf of 12.0 V.…
A: Given that-Total emf created by battery, ETotal= 12.0 VoltNumber of lead-acid , n=6Total emf of…
Q: Which of the following is an installation requirement for the grounding electrode conductor in a…
A: The demand for computer network installations has increased the opportunity for electrical…
Q: 1. If the filament resistance in a car headlamp is 30, how many Amps does it draw when connected to…
A: Ohm's law states that the current through a conductor between two pointsis directly proprotional to…
Q: A researcher measures the thickness of a layer of benzene (n = 1.50) floating on water by shining…
A:
Q: Q1: (Planar mechanism) For the planar mechanism of figure below, link 4 serves as slider support…
A: The combination of two or more machine components intended to transfer a force or motion across…
Q: Two planets are on a collision course, heading directly towards each other at 0.250c. A spaceship…
A: We know that-Relativistic velocity of transformation is, u= v+u'1+u'vc2 ..... Equation 01where,…
Q: Find the eigenvalues and the eigenstates of S
A: Solution: The spin-y (Sy) operator is given by, sy=♄20-ii0 The eigenvalue of the above operations…
Q: An emf of 9.7 x 10-3 V is induced in a coil while the current in a nearby coil is decreasing at a…
A: Given that-EMF 'ε'= 9.7×10-3 Vdidt= 2.7 A/sEMF of a coil 'ε'= -M×dIdt .... eqn 1after arranging…
Q: Q.7 a) Ring and number the junctions of component in the below circuit. R2 R1 R4 Output Q2 Input D1.…
A: Given that the circuit diagram can be given as,
Q: There are 3 parallel wires which are spaced D=4cm. They lie on the same plane. Each Carries a steady…
A: A current carrying wire exerts a magnetic force per unit length on another current current carrying…
Q: Ignore the coefficients. What is the final state of the particle after using the operator below?…
A: Given : Initial state of the particle is ψn We have to find the state of the particle after using…
Q: A coil has 2.55-Q resistance and 121-mH inductance. If the current is 3.00 A and is increasing at a…
A: Given that :Resistance of coil, R= 2.25ΩInductance of coil, L= 121 mH…
Q: 18) Find the total binding energy of 208Pb
A: The subatomic particles inside a nucleus are called nucleons. The two nucleons are protons and…
Q: At At the intersection, a yellow subcompact car with a mass of 950 kg traveling east collides with a…
A: An inelastic collision is a collision in which kinetic energy is not conserved due to the action of…
Q: Find the maximum velocity of photoelectrons ejected by an 80-nm radiation, if the work function of…
A: Given that-Wavelength of incident radiation= 80 nmWork-function of the photoelectrode is 4.73 eVWe…
Q: A circular loop in the xy plane is stationary in a uniform magnetic field, which points into the…
A: When there is motion of conductor in magnetic field, an emf is set. A current starts flowing. This…
Q: 1. If the filament resistance in a car headlamp is 30, how many Amps does it draw when connected to…
A: Ohm's law states that the current through a conductor between two pointsis directly proprotional to…
Q: ges by a 5.95 nm X-ray, which experiences an increase in wavelength of 1.97 pm. How fast is the…
A: Scattered electron.
Q: The wavefunction of a 1D PIB is given by: 4₁-415567 sin( [π/a] x) what is the box length in pm? O a.…
A:
Q: 9. The resultant state for the quantum circuit shown in (Figure 5) is: |0) H X |1) |0) Figure 5:…
A: The matrix form of Hadamard gate (H), X gate, and Z gate is
Q: The energy of the election in the ground state of hydrogen alom is -13.6 eV. Find its energy in the…
A: We need to find-(1) Energy in 2nd orbit.(2) Energy in 3rd orbit.(3) Frequency of radiation.Given…
Step by step
Solved in 2 steps with 1 images
