Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 53QAP
Related questions
Question
Identify the structure of the compound a with a formula of C3H6O2 having the following IR, and 1H NMR spectra (integrals and multiplicity shown in the boxes: s-singlet, d-doublet, t-triplet, q-quartet, etc.). Label the spectra with proper information you deduced (
Compound formula. C3H6O2
Questions to help you decide
- What is the element of unsaturation of the molecular formula? Show the equation you use:
- What are the functional groups present in this molecule? Show all of them belowDraw at least two possible structures that have the .
- required element of unsaturation as well as the observed functional groups:
-
Based on the 1H NMR above, what molecular fragmentations do you see:
-
Draw your final decision of the structure below. Is this one of the structures in your answer 5C?
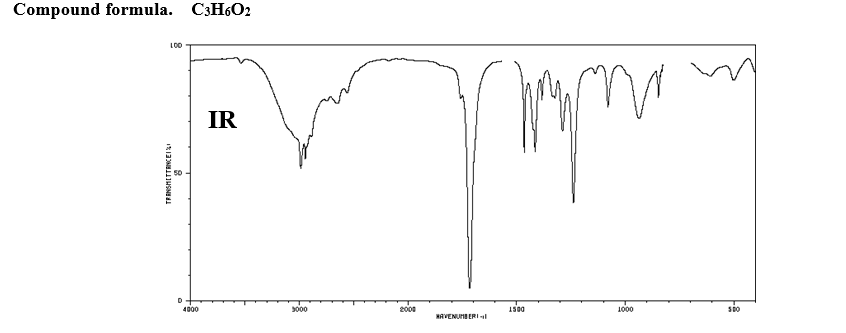
Transcribed Image Text:Compound formula. C3H6O2
L00
IR
4000
5000
2000
1500
1000
MAVENUMB ERI l
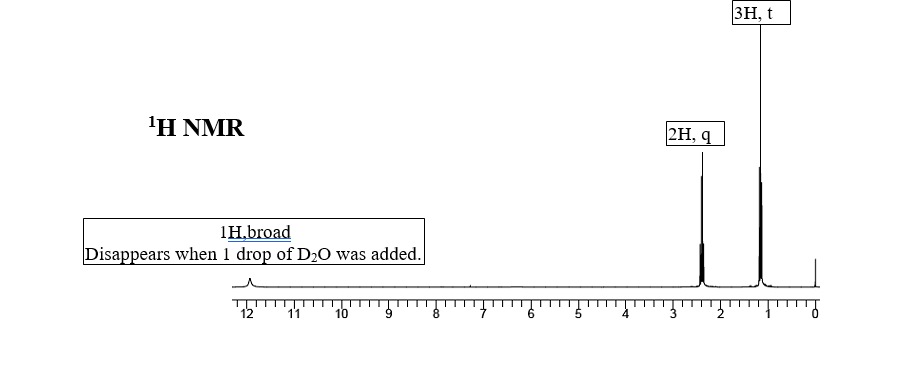
Transcribed Image Text:3H, t
ΙΗ ΝMR
2H, q
1H,broad
Disappears when 1 drop of D20 was added.
12
11
10
8.
5
4
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning