
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
PROBLEM 29) Can you explain this problem and solve it correctly, please!
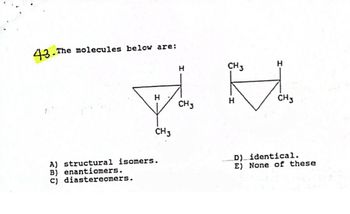
Transcribed Image Text:43. The
The molecules below are:
H
CH 3
H
A) structural isomers.
B) enantiomers.
(c) diastereomers.
H
H
CH 3
CH 3
CH 3
D) identical.
E) None of these
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Determine whether each pair of molecules represent: (a) identical compounds; (b) constitutional (structural) isomers, (c) enantiomers, or (d) diastereomers. Thank you.arrow_forwardFO 23: Identify and draw conformers. NH₂ NH2 NH2 NH2 NH2 NH2 1 NH2 NH2 4 2 3 (a) List any two structures that are related as conformers. Answer by listing exactly two numbers. (b) Draw one conformer of the new structure shown here: 1 and 3 (b) OH HO OHarrow_forwardWhich of the following line drawings represents the correct stereochemistry o molecule shown in the Newman projection below? E + Н. H I CH₂CH3 LOH H CH3 OH OH || IV OH 5.... OH A) I B) II C) III D) IV Karrow_forward
- What term describes the structural relationship between (2R 3R,4S)-2,3,4-trichloroheptane and (2R 3R,4R)-2,3,4-trichloroheptane? O diastereomers O not isomers O conformers constitutional isomers O enantiomersarrow_forwardd) Molecular relationships; identify whether the compounds in each group are const. isomers, conformers, enantiomers, diastereomers, identical, meso or none of the above. ОН ОН H3C Br H3C LF H Br CH3 HO CI Br Br CI NH NHarrow_forward129 Identify the relationship in each of the following pairs. Do the drawings represent consti- tutional isomers or stereoisomers, or are they just different ways of drawing the same compound? Ir they are stereoisomers, are they enantiomers or diastereomers? (Molecular models may prove useful in this problem.) CH) (a) C-CH,Br and c-CH,OH HO Br CH, H. Br H, (b) C-Br and CH, CH,CH, CH,CH, CH, Hc H C-CH,CH, (c) C-Br and CH,CH, Br CH, and Br CH,CH, CH,OH CH,OH (e) HOH ČHOH and но- ČH,OH H,C (r) and For Problem No. 7.29 (a) to (f), IDENTIFY the given pair of structures as described below and WRITE: EN if the pair represents ENANTIOMERS DI if the pair represents DIASTEREOMERS co if the pair represents CONSTITUTIONAL isomers ID if the two molecules represent IDENTICAL compoundsarrow_forward
- Please don't provide handwriting solutionarrow_forward4. Isomers and Stereocenters ( (a) Assign each set of molecules as constitutional isomers, conformational isomers, enantiomers, diastereomers, or identical. Please write the full name for the type. Molecules Type (b) H₂Ca HgC.. CH3 OCH₂ CH3 af OCH, SP H₂C**** H₂C H₂C NGOCH, H.C_OCKS OCH₂ CH₂ H₁C HC H*** CI H H CI Br H3C CH₂ Br (d) E) (0) For each of the following molecules, write the number of stereocenters and circle the center of any stereocenters. (i) (ii) H₂C (онт (eg 5) (b) -CH3 Isegna CH3 on mots eno H₂C CH3arrow_forwardFor the pair of structures indicate what type of isomeric relationship they have at room temperature. The possibilities are structural isomers, conformational isomers, enantiomers, diastereomers, and meso compound. O |||||OH CH3 "1110 ...IOH CH3 AV Aarrow_forward
- What is the relationship between these structures? H3CH2C OH CH3 HỌC HỒ CH(CH3)2 (A) conformers (C) enantiomers HO CH(CH3)2 CH2CH3 H3C OH CH3 (B) diastereomers (D) identicalarrow_forward4. For each pair Stute whetter th two are DJ HHe Same moleclle, b) defferent compounds that ave not isomers, C) constitutonal isomers dl diastereomers, or e enantiomers, SH a) Hs Octs Br CH3 iBr b)arrow_forwardwhat is the line structure?' a) CH3CH2CH2OH b) CH2CHCH2CH3 c) (CH3)2CHCH2CH2NH2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning