55 Cs 112.9055 Fr 87.62 8.905 91.22 92.9054 905.94 36 57 72 73 74 Ba La Hf Ta W 117.34 138.0055 17840 1809479 18385 88 89 104 105 106 Ra Ac Rf Db Sg 226.00.54 (227) (257) (266) Q) 39 60 Ce Pr Nd 140 12 140.9077 144.24 90 91 92 Th Pa U 252.081 231.05.59 23.03 7 Select one: O a. K< Fe < Li O b. Rb> Na > Be O c. Fe
55 Cs 112.9055 Fr 87.62 8.905 91.22 92.9054 905.94 36 57 72 73 74 Ba La Hf Ta W 117.34 138.0055 17840 1809479 18385 88 89 104 105 106 Ra Ac Rf Db Sg 226.00.54 (227) (257) (266) Q) 39 60 Ce Pr Nd 140 12 140.9077 144.24 90 91 92 Th Pa U 252.081 231.05.59 23.03 7 Select one: O a. K< Fe < Li O b. Rb> Na > Be O c. Fe
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 76E: A 230-L sample of a colorless gas at STP decomposed to give 230 L of N2 and 1.25 L of O2 at STP....
Related questions
Question
arrangement by using increasing atomic radius pleaseeee
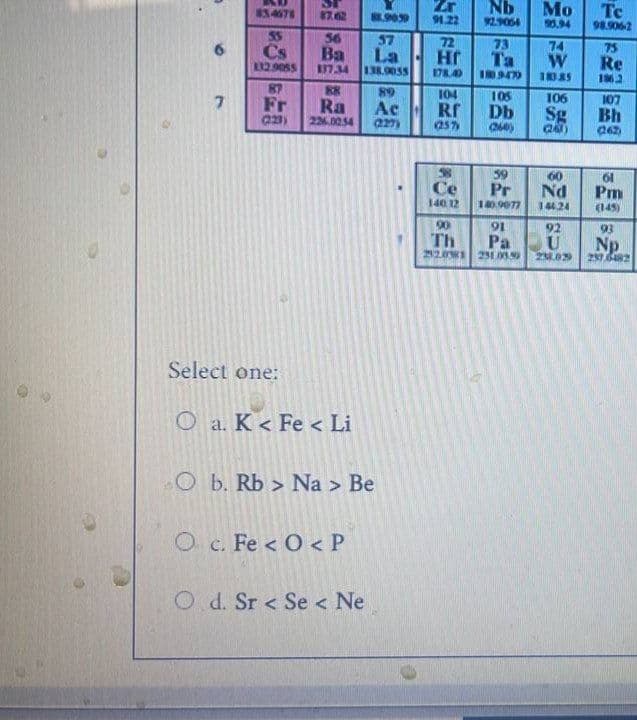
Transcribed Image Text:ST
87.62
56
Ba
137.34
88
Ra
226.00.54
834678
Cs
112.9055
87
Fr
(223)
Select one:
O a. K < Fe < Li
O b. Rb> Na > Be
O c. Fe <O< P
Od. Sr < Se < Ne
7
88.90.59
57
La
138.9055
89
Ac
(227)
Zr
91.22
Nb Mo
92.9064
90.94
72
73
74
Hr
Ta
W
178.40 180.947
18385
104
105
106
Rf
Db
Sg
(25%)
(260)
(263)
59
60
Ce
Pr
Nd
140 12
140.9077
144.24
90
91
92
Th
Pa
U
22.01 231.05.99 23.03
Tc
98.906-2
75
Re
1962
107
Bh
(262)
61
Pm
(145)
93
Np
237.6482
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
