
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN: 9781285866932
Author: Lauralee Sherwood
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
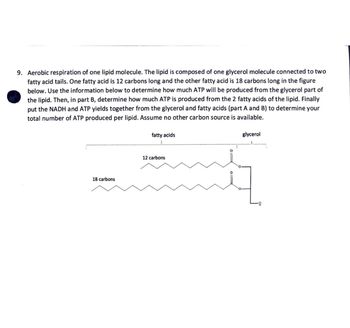
Transcribed Image Text:9. Aerobic respiration of one lipid molecule. The lipid is composed of one glycerol molecule connected to two
fatty acid tails. One fatty acid is 12 carbons long and the other fatty acid is 18 carbons long in the figure
below. Use the information below to determine how much ATP will be produced from the glycerol part of
the lipid. Then, in part B, determine how much ATP is produced from the 2 fatty acids of the lipid. Finally
put the NADH and ATP yields together from the glycerol and fatty acids (part A and B) to determine your
total number of ATP produced per lipid. Assume no other carbon source is available.
fatty acids
glycerol
18 carbons
12 carbons
0=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- 9. Aerobic respiration of one lipid molecule. The lipid is composed of one glycerol molecule connected to two fatty acid tails. One fatty acid is 12 carbons long and the other fatty acid is 18 carbons long in the figure below. Use the information below to determine how much ATP will be produced from the glycerol part of the lipid. Then, in part B, determine how much ATP is produced from the 2 fatty acids of the lipid. Finally put the NADH and ATP yields together from the glycerol and fatty acids (part A and B) to determine your total number of ATP produced per lipid. Assume no other carbon source is available. 18 carbons fatty acids 12 carbons 9 glycerol A. Glycerol is broken down to glyceraldehyde 3-phosphate, a glycolysis intermediate via the following pathway shown in the figure below. Notice this process costs one ATP but generates one FADH2. Continue generating ATP with glyceraldehyde-3-phosphate using the standard pathway and aerobic respiration. glycerol glycerol-3- phosphate…arrow_forwardfill in the blanks with the missing structures and give namesarrow_forwardFigure 2.17 Fatty acids. Double bonds in the tails are highlighted in red. A. The tail of stearic acid is fully saturated with hydrogen atoms. B. Linoleic acid, with two double bonds, is unsaturated. The first double bond occurs at the sixth carbon from the end of the tail, so linoleic acid is called an omega-6 fatty acid. Omega-6 and C. omega-3 fatty acids are essential fatty acids, which means your body does not make them and they must come from food. D. The hydrogen atoms around the double bond in oleic acid are on the same side of the tail. Most other naturally occurring unsaturated fatty acids have these cis bonds. E. Hydrogenation creates abundant trans bonds, with hydrogen atoms on opposite sides of the tail. Figure It Out: Are the double bonds in linolenic acid cis or trans?arrow_forward
- Name three kinds of carbohydrates that can be built using only glucose monomers.arrow_forwardFigure 7.12 Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the pH of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis?arrow_forwardEnergetic of Fructose-1 ,6-bis P Hydrolysis (Integrates with Chapter 3.) The standard free energy change (G) for hydrolysis of fructose-1. 6-bisphosphate (FBP) to fructose-S-phosphate (F-6-P) and P: is -16.7 KJ/mol: FBP + H2O fructose-6-P + Pi The standard free energy change (G) for ATP hydrolysis is -30.5 KJ/mol: ATP + H2O ADP + Pj What is the standard free energy change for the phosphofructokinase reaction: ATP + fructose-6-P ADP + FBP b. What is the equilibrium constant for this reaction? c. Assuming the intracellular concentrations of [ATP] and (ADP] are maintained constant at 4 mM and 1.6 mM, respectively, in a rat liver cell, what will be the ratio of [FBP]/[fructose-6-P] when the phosphofructokinase reaction reaches equilibrium?arrow_forward
- Which of the following reaction pathways is not part of the second stage of aerobic respiration? a. electron transfer phosphorylation b. acetyl-CoA formation c. Krebs cycle d. glycolysis e. a and darrow_forwardFigure 27.3 illustrates the response of R (ATP-regenerating) and U (ATP-utilizing) enzymes to energy charge. a. Would hexokinase be an R enzyme or a U enzyme? Would glutamine: PRPP amidotransferase, the second enzyme in purine biosynthesis, be an R enzyme or a U enzyme? b. If energy charge = 0.5: Is the activity of hexokinase high or low? Is ribose-5-P pyrophosphokinase activity high or low? c. If energy charge = 0.95: Is the activity of hexokinase high or low? Is ribose-5-P pyrophosphokinase activity high or low?arrow_forward_______ is (are) a good source of omega-3 fatty acids that can reduce the risk of heart disease. a. Oatmeal b. Legumes c. Fish d. Com syruparrow_forward
- Glycolysis results in the production of two ________ molecules from a single molecule of glucose. In the absence of ________, the end product of glycolysis is ________. acetyl CoA, pyruvate, lactate ATP, carbon, pyruvate pyruvate, oxygen, lactate pyruvate, carbon, acetyl CoAarrow_forwardWhen glucose reacts with ATP to form glucose-6-phosphate: a. the synthesis of glucose-6-phosphate is exergonic. b. ADP is at a higher energy level than ATP. c. glucose-6-phosphate is at a higher energy level than glucose. d. because ATP donates a phosphate to glucose, this is not a coupled reaction. e. the reaction is spontaneous.arrow_forwardAfter the citric acid cycle reactions run _________, one six-carbon glucose molecule has been completely broken down to CO2. a. once b. twice c. six times d. twelve timesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning


Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax