A 22-cm-diameter cylinder that is 45 cm long contains 46 g of oxygen gas at 20°C. You may want to review (Pages 500 - 502). For general problem-solving tips and strategies for this topic, you may want to view a Video Tutor Solution of Air in warmed tire. How many moles of oxygen are in the cylinder? Express your answer using two significant figures. 15. ΑΣΦ n = Submit Part B How many oxygen molecules are in the cylinder? Express your answer using two significant figures. 5 ΑΣΦ N = Request Answer Submit Request Answer ? ? mol molecules
A 22-cm-diameter cylinder that is 45 cm long contains 46 g of oxygen gas at 20°C. You may want to review (Pages 500 - 502). For general problem-solving tips and strategies for this topic, you may want to view a Video Tutor Solution of Air in warmed tire. How many moles of oxygen are in the cylinder? Express your answer using two significant figures. 15. ΑΣΦ n = Submit Part B How many oxygen molecules are in the cylinder? Express your answer using two significant figures. 5 ΑΣΦ N = Request Answer Submit Request Answer ? ? mol molecules
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 49P: The height of the Washington Monument is measured to be 170.00 m on a day when the temperature is...
Related questions
Question
PART A AND PART B
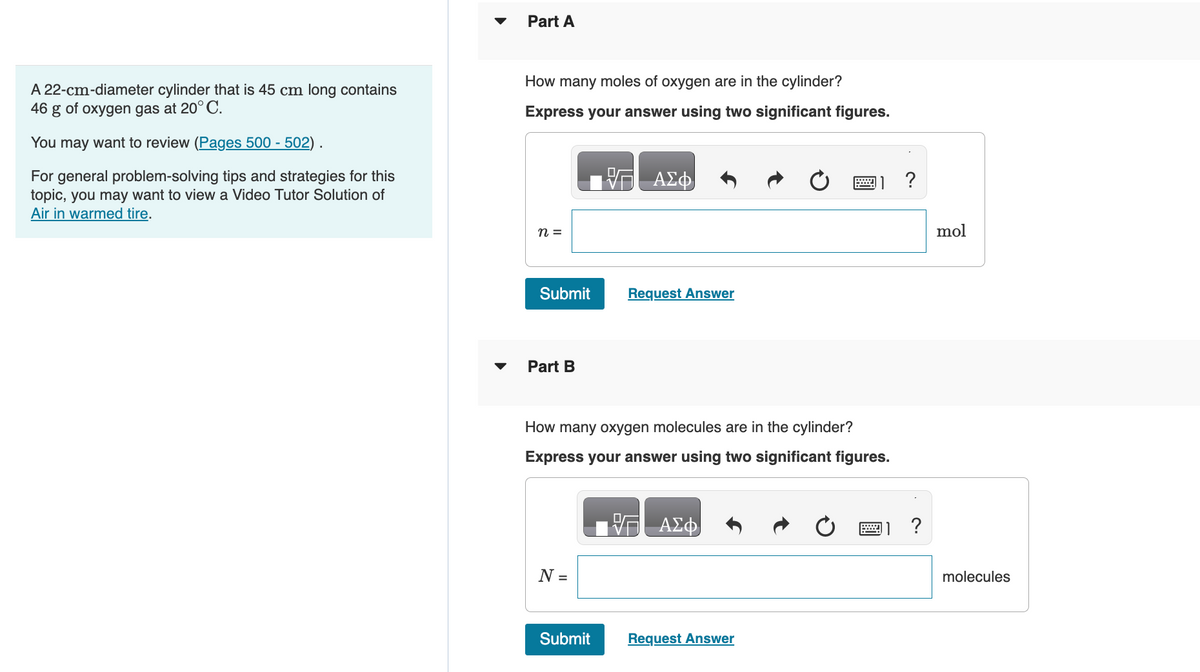
Transcribed Image Text:A 22-cm-diameter cylinder that is 45 cm long contains
46
g of oxygen gas at 20° C.
You may want to review (Pages 500 - 502).
For general problem-solving tips and strategies for this
topic, you may want to view a Video Tutor Solution of
Air in warmed tire.
▼
Part A
How many moles of oxygen are in the cylinder?
Express your answer using two significant figures.
n =
Submit
Part B
N =
ΑΣΦ
Submit
Request Answer
How many oxygen molecules are in the cylinder?
Express your answer using two significant figures.
V— ΑΣΦ
*****
Request Answer
?
?
mol
molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning