A balloon is filled with helium gas. For the question s(11, 12) that follow, select the letter of the balloon diagram that corresponds to the given change in conditions. Initial Volume B C 9999 D 11) The balloon is put into a chamber whose pressure is less than the atmospheric pressure atmospheric temperature. A) A B) B C) C D) A and B E) B and C 12) The temperature is changed from 50 °C to -150 °C at constant pressure. A) A B) B C) C D) A and B E) B and C and at
A balloon is filled with helium gas. For the question s(11, 12) that follow, select the letter of the balloon diagram that corresponds to the given change in conditions. Initial Volume B C 9999 D 11) The balloon is put into a chamber whose pressure is less than the atmospheric pressure atmospheric temperature. A) A B) B C) C D) A and B E) B and C 12) The temperature is changed from 50 °C to -150 °C at constant pressure. A) A B) B C) C D) A and B E) B and C and at
Chapter5: Gases
Section: Chapter Questions
Problem 26Q: Consider two different containers, each filled with 2 moles of Ne(g). One of the containers is rigid...
Related questions
Question
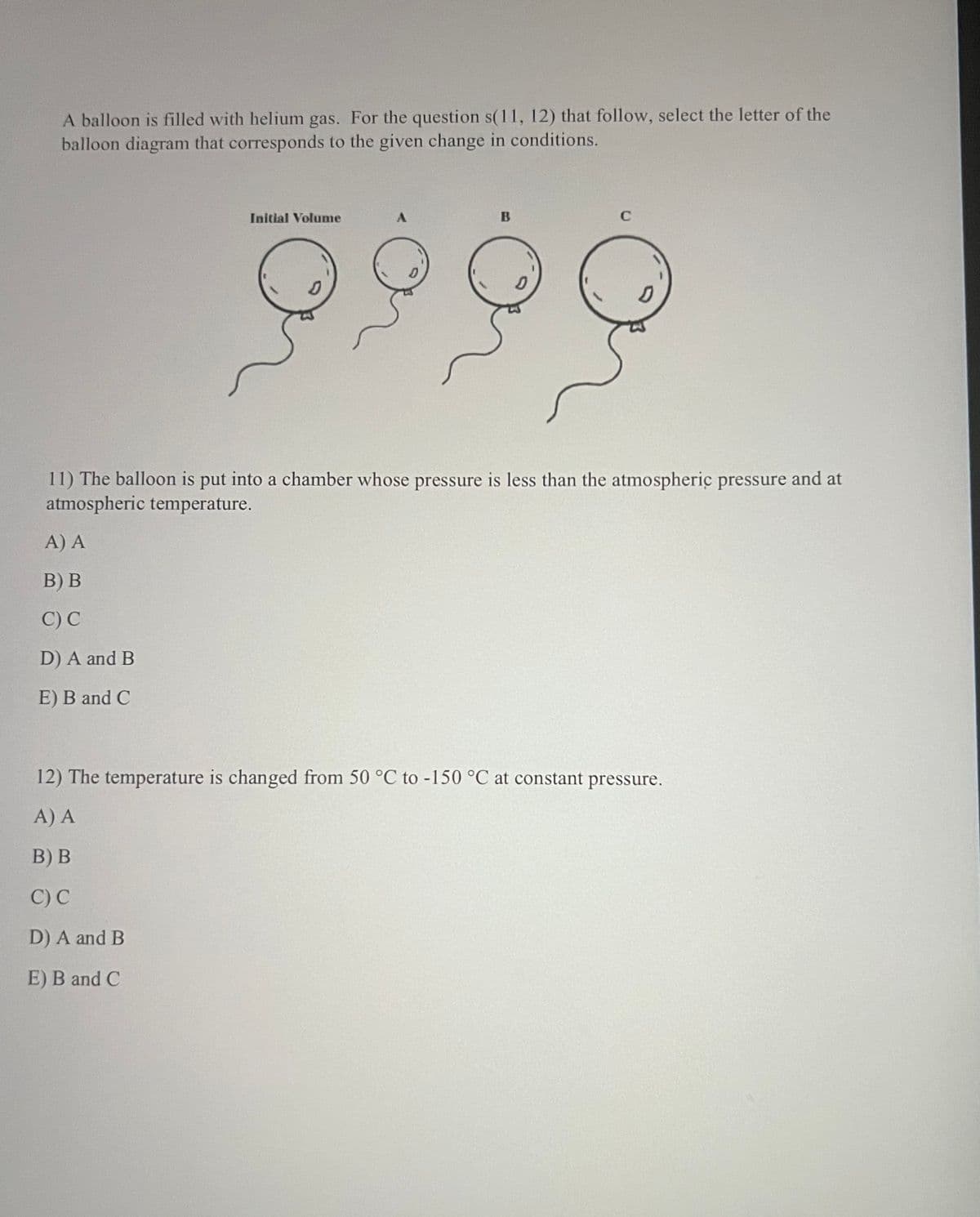
Transcribed Image Text:A balloon is filled with helium gas. For the question s(11, 12) that follow, select the letter of the
balloon diagram that corresponds to the given change in conditions.
Initial Volume
B
C
9999
D
11) The balloon is put into a chamber whose pressure is less than the atmospheric pressure
atmospheric temperature.
A) A
B) B
C) C
D) A and B
E) B and C
12) The temperature is changed from 50 °C to -150 °C at constant pressure.
A) A
B) B
C) C
D) A and B
E) B and C
and at
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
