
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please help me with #2b & #3 using the data.
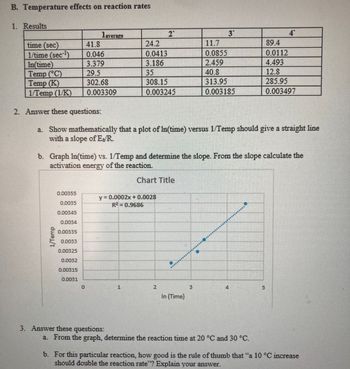
Transcribed Image Text:B. Temperature effects on reaction rates
1. Results
1average
2
3"
4
time (sec)
41.8
24.2
11.7
89.4
1/time (sec-¹)
0.046
0.0413
0.0855
0.0112
In(time)
3.379
3.186
2.459
4.493
Temp (°C)
29.5
35
40.8
12.8
Temp (K)
302.68
308.15
313.95
285.95
1/Temp (1/K)
0.003309
0.003245
0.003185
0.003497
2. Answer these questions:
a. Show mathematically that a plot of In(time) versus 1/Temp should give a straight line
with a slope of E/R.
b. Graph In(time) vs. 1/Temp and determine the slope. From the slope calculate the
activation energy of the reaction.
1/Temp
0.00355
0.0035
0.00345
0.0034
0.00335
0.0033
0.00325
0.0032
0.00315
Chart Title
y = 0.0002x + 0.0028
R² = 0.9686
0.0031
0
1
N
In (Time)
E
4
5
3. Answer these questions:
a. From the graph, determine the reaction time at 20 °C and 30 °C.
b. For this particular reaction, how good is the rule of thumb that "a 10 °C increase
should double the reaction rate"? Explain your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 7-12 Two kinds of gas molecules are reacted at a set temperature. The gases are blown into the reaction vessel from two tubes. In setup A, the two tubes are aligned parallel to each other; in setup B, they are 900 to each other; and in setup C, they are aligned directly opposite each other. Which setup would yield the most effective collisions?arrow_forwardThe following series of diagrams represent the reaction XY followed over a period of time. The X molecules are red and the Y molecules are green. At the end of the time period depicted, has the reaction system reached equilibrium? Justify your answer with a one-sentence explanation.arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forward
- Help with the questions like the objectives and the hypothesis and all of thosearrow_forwardKINETICS AND EQUILIBRIUM Using Le Chatelier's Principle to predict the result of changing... Methane and water react to form hydrogen and carbon monoxide, like this: CH_(g)+H,O(g) …+ 3H,(g)+CO(g) perturbation The reaction is endothermic. Suppose a mixture of CH4, H₂O, H₂ and CO has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. change in composition The temperature is raised. The pressure of CO will ? The temperature is lowered. The pressure of CH4 will? ? go up. go down. not change. shift in equilibrium to the right to the left O (none) to the right to the left O (none) X 0/5 Garrow_forwardFOR THE REACTION CH4 (G) + 2 O2 (G) <- -> CO2 (G) + 2 H2O (L) SHOW W ARROW HOW TO REDUCE THE FOLLOWING STRESSES TO THE REACTION (A) DECREASE THE TOTAL VOLUME OF THE SYSTEM (B) ADDITIONAL O2(G) IS ADDED TO THE SYSTEMarrow_forward
- Hi! Can someone answer Learning Task 1? Explain the required answers briefly. Thanks !arrow_forward1 10:44 7.5 student.pdf Approximation Kule: When can it be made? An approximation may be made when the initial concentration of the reactants is more than 1000x greater than the Keq. When this is the case, the change in the concentration of the reactants will be negligible at equilibrium. Thus the initial concentrations of the reactants can be used to represent the equilibrium centration of the reactants: i.e. [initial] reactants= [eq] reactants Sample Problem The atmosphere contains large amounts of nitrogen and oxygen gases which do not react under ordinary conditions. However, they do react under extreme conditions such as those produced by a flash of lightning or inside the running of a car engine. The production of nitrogen oxides from these exhaust gases are a huge pollution problem. If a chemist puts 0.085 mol of nitrogen and 0.038 mol of oxygen gas in a rigid 1.5 L cylinder. What is the concentration of nitrogen monoxide gas NO (g) in the mixture at equilibrium? N2 (9) + O2( 2…arrow_forwardThis is no help to me because it is not an explanasion USING the collision theoryarrow_forward
- Calc solubility 2arrow_forwardLequel des composés ci-dessous pourrait être utilisé comme réactif de départ dans la réaction suivante?arrow_forward7) For the reaction CO(g) + 3 H2(g) = H2O(g) + CH4(g), Kc = 190 at 1000 K. If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.036 M, [H2] = 0.045, [H2O] = 0.020, 7) %3D %3D %3D and [CH4] = 0.031, in which direction will a reaction occur and why? A) it is at equilibrium because Q = 190 B) toward products because Q = 4.1 C) toward reactants because Q = 0.24 D) toward products because Q = 0.38 %3D %3D %3D %3D E) toward reactants because Q = 61 %3Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning