Calculate AG' at 25 °C for the reaction: ATP + pyruvate = ADP + phosphoenolpyruvate Calculate Keq at 25 °C for the reaction: ATP + pyruvate= ADP + phosphoenolpyruvate What is the equilibrium ratio of pyruvate to phosphoenolpyruvate (PEP) if the ratio of ATP to ADP is 10? Calculate AGo for the isomerization of glucose 6-phosphate to glucose 1-phosphate at 25 °C. What is the equilibrium ratio of glucose 6-phosphate (G6P) to glucose 1-phosphate (G1P) at 25 °C? AGO = Kéq= 3 x10 31.4 AG⁰' = [pyruvate] 3 x104 [PEP] [G6P] [GIP] Incorrect -6 6 X10-² Incorrect 0.85 Incorrect kJ mol-¹ kJ mol-1
Calculate AG' at 25 °C for the reaction: ATP + pyruvate = ADP + phosphoenolpyruvate Calculate Keq at 25 °C for the reaction: ATP + pyruvate= ADP + phosphoenolpyruvate What is the equilibrium ratio of pyruvate to phosphoenolpyruvate (PEP) if the ratio of ATP to ADP is 10? Calculate AGo for the isomerization of glucose 6-phosphate to glucose 1-phosphate at 25 °C. What is the equilibrium ratio of glucose 6-phosphate (G6P) to glucose 1-phosphate (G1P) at 25 °C? AGO = Kéq= 3 x10 31.4 AG⁰' = [pyruvate] 3 x104 [PEP] [G6P] [GIP] Incorrect -6 6 X10-² Incorrect 0.85 Incorrect kJ mol-¹ kJ mol-1
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
![Calculate AG°' at 25 °C for the reaction:
ATP + pyruvate = ADP + phosphoenolpyruvate
Calculate Keq at 25 °C for the reaction:
ATP + pyruvate = ADP + phosphoenolpyruvate
What is the equilibrium ratio of pyruvate to
phosphoenolpyruvate (PEP) if the ratio of ATP to ADP is 10?
Calculate AG' for the isomerization of glucose 6-phosphate
to glucose 1-phosphate at 25 °C.
What is the equilibrium ratio of glucose 6-phosphate (G6P)
to glucose 1-phosphate (G1P) at 25 °C?
AGO! =
Keq=
[pyruvate]
[PEP]
AGO!
II
[G6P]
[G1P]
31.4
3 ×10-6
Incorrect
3 x104
6 X10-2
Incorrect
0.85
Incorrect
kJ mol-¹
kJ mol-¹](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F049a8034-a0ab-4b8d-b3d1-3977f5f9e464%2F0cef294c-7a7b-4d2c-91ad-81033e667b16%2Fx2j2cc4_processed.png&w=3840&q=75)
Transcribed Image Text:Calculate AG°' at 25 °C for the reaction:
ATP + pyruvate = ADP + phosphoenolpyruvate
Calculate Keq at 25 °C for the reaction:
ATP + pyruvate = ADP + phosphoenolpyruvate
What is the equilibrium ratio of pyruvate to
phosphoenolpyruvate (PEP) if the ratio of ATP to ADP is 10?
Calculate AG' for the isomerization of glucose 6-phosphate
to glucose 1-phosphate at 25 °C.
What is the equilibrium ratio of glucose 6-phosphate (G6P)
to glucose 1-phosphate (G1P) at 25 °C?
AGO! =
Keq=
[pyruvate]
[PEP]
AGO!
II
[G6P]
[G1P]
31.4
3 ×10-6
Incorrect
3 x104
6 X10-2
Incorrect
0.85
Incorrect
kJ mol-¹
kJ mol-¹
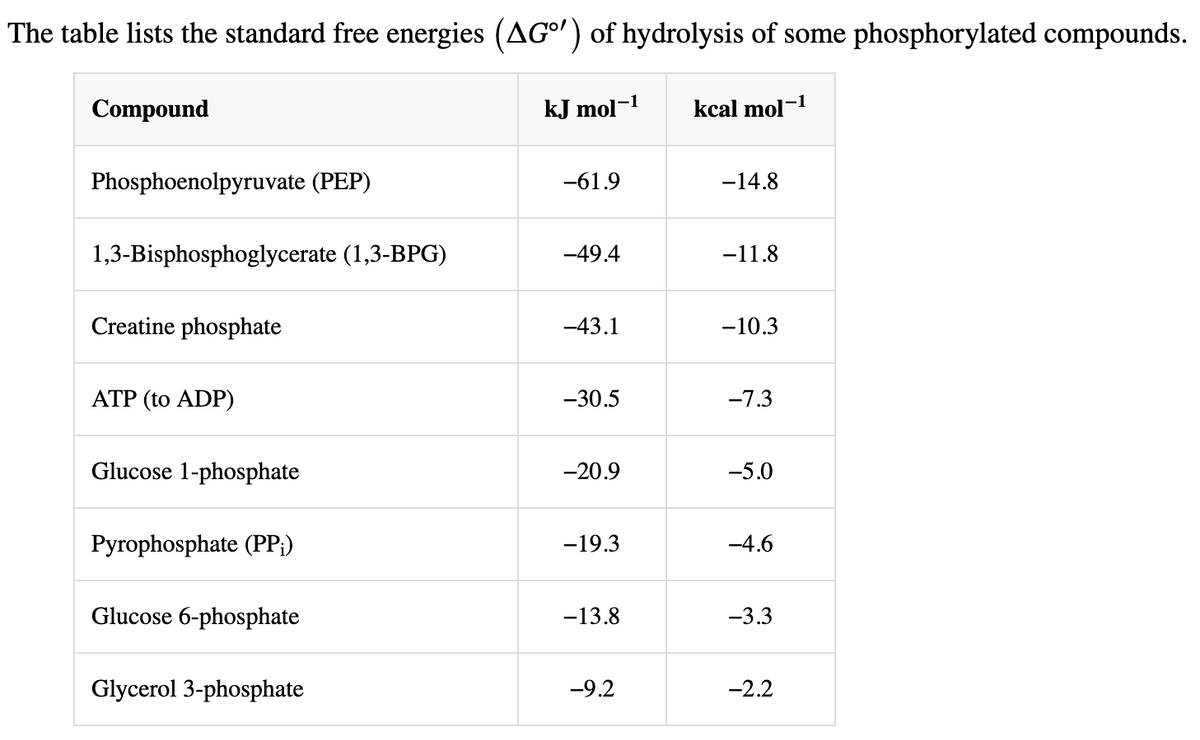
Transcribed Image Text:The table lists the standard free energies (AG°') of hydrolysis of some phosphorylated compounds.
Compound
Phosphoenolpyruvate (PEP)
1,3-Bisphosphoglycerate (1,3-BPG)
Creatine phosphate
ATP (to ADP)
Glucose 1-phosphate
Pyrophosphate (PP;)
Glucose 6-phosphate
Glycerol 3-phosphate
kJ mol-¹
-61.9
-49.4
-43.1
-30.5
-20.9
-19.3
-13.8
-9.2
kcal mol-1
-14.8
-11.8
-10.3
-7.3
-5.0
-4.6
-3.3
-2.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 7 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON