
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Problem 35 can u explain and solve thanks april 24
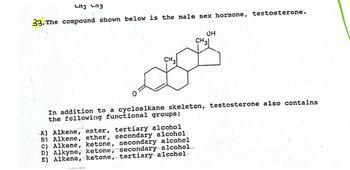
Transcribed Image Text:CH3 CH3
33. The compound shown below is the male sex hormone, testosterone.
OH
CH 3
CH3
In addition to a cycloalkane skeleton, testosterone also contains
the following functional groups:
A) Alkene, ester, tertiary alcohol
B) Alkene, ether, secondary alcohol
C) Alkene, ketone, secondary alcohol
D) Alkyne, ketone, secondary alcohol
E) Alkene, ketone, tertiary alcohol-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- In what functional groupings are the structures composed? ▸ B H&CH=CH₂ A) A ketone and an alkyne A) An alcohol and an alkyne B) A ketone and an ether B) An Aldehyde and a alkene A) A ketone and an alkene B) An alcohol and an alkene A) An Aldehyde and a ketone B) A ketone and an alkene CH3-CEC-CH₂OH ◆- Which type of bonding between carbon atoms in organic molecules is NOT typical? X₁ ◆ A functional component called the carbonyl group can be found in a wide range of substances. What's the carbonyl group's basic structure? CH A) -C- 8 C)--0)-C30 e) -4² ◆Which of the following statements about the two compounds is untrue? OH CH₂ - CH₂ - CH₂ Compound A A) Compound B has stronger intermolecular forces than compound A. B) Compound A and compound B are both organic compounds. C) Compound B has a higher boiling point than compound A. D)Compound A is Likely a gas at room temperature and compound B is likely a liquid at room temperature. E) Compound A and compound B are both hydrocarbons ◆ which…arrow_forward+ Complete the table Molecular Formula Structural Formula Condensed Formula Skeletal Formula Types of Hydrocarbon (Alkane, Alkene, Alkyne) CH;CH;CHCHCH3 CH;CH=CHCH3 CH6 Note: General Formula of Alkane (C,H2n-2), Alkene (C,H2), Alkyne (C,H2n-2). Where n is the number of C atom in the formula. Alkane (C-C), Alkene (C=C) and Alkyne (C=C)arrow_forwardBb.35.arrow_forward
- Here is the chemical structure of methyl isopropanoate: CH;-0-C-CH-CH; dlo CH, Decide whether each molecule in the table below is another molecule of methyl isopropanoate, a molecule of an isomer of methyl isopropanoate, or a molecule of an entirely different compound. molecule relationship to methyl isopropanoate C-CH, (Choose one) another molecule of methyl isopropanoate molecule of an isormer of methyl isopropanoate a molecule of a different compound CH,-CH-O- CH; (Choose one) CH, -CH-C–CH, CH O. CH, (Choose one) -C-CH CH,arrow_forwardidentify each functional group in the molecules shownarrow_forwardPlease classify each compound as saturated or unsaturated. Identify each as alkane, an alkene, or an alkyne.arrow_forward
- (b) H₂C 4-ethy-3-methylnonane CH₂ Marvin JS H₂ X Write the structural formulas for the following hydrocarbons. (c) 2,2-dimethylbutane Helparrow_forwardClassify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications: alkane cycloalkane aromatic hydrocarbon alkene cycloalkene alkyne evcloalkyne (d) H,-C=C-CH CH, —CH, —CH, —сH CHCH2 (f) CCH - СНC(CH), (h) - CH2CH3 (i)arrow_forwardSafari File Edit View History Bookmarks Window Help 36% O A АBС Thu 3:08 PM 5. The general formula of alkene is C„H2n. (a) The relative molecular mass of an alkene X is 70.0 . Find out the molecular formula of X. (Relative atomic masses: C = 12.0, H = 1.0) (b) X is a straight chain alkene showing cis-trans isomerism. (i) Give the structural formula of X. (ii) Draw a 3-dimension diagram of the trans-isomer of X.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co