Complete combustion of methane = the mole-to-mole stoichiometry among the ts and products. The molar mass (in ) can be used as the conversion factor n moles and the mass of a substance. the balanced equation and molar masses used in conjunction with one another to te the masses involved in a reaction. When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. TH unbalanced equation for this reaction is CH4 (g) + O2 (g)–→CO2(g)+H2O(g) This type of reaction is referred to as a complete combustion reaction. Part A What coefficients are needed to balance the equation for the complete combustion of meth the coefficients in the order CH4, O2, CO2, and H2O, respectively. Express your answer as four integers, separated by commas (e.g., 1,2,3,4).
Complete combustion of methane = the mole-to-mole stoichiometry among the ts and products. The molar mass (in ) can be used as the conversion factor n moles and the mass of a substance. the balanced equation and molar masses used in conjunction with one another to te the masses involved in a reaction. When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. TH unbalanced equation for this reaction is CH4 (g) + O2 (g)–→CO2(g)+H2O(g) This type of reaction is referred to as a complete combustion reaction. Part A What coefficients are needed to balance the equation for the complete combustion of meth the coefficients in the order CH4, O2, CO2, and H2O, respectively. Express your answer as four integers, separated by commas (e.g., 1,2,3,4).
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 1STP
Related questions
Question
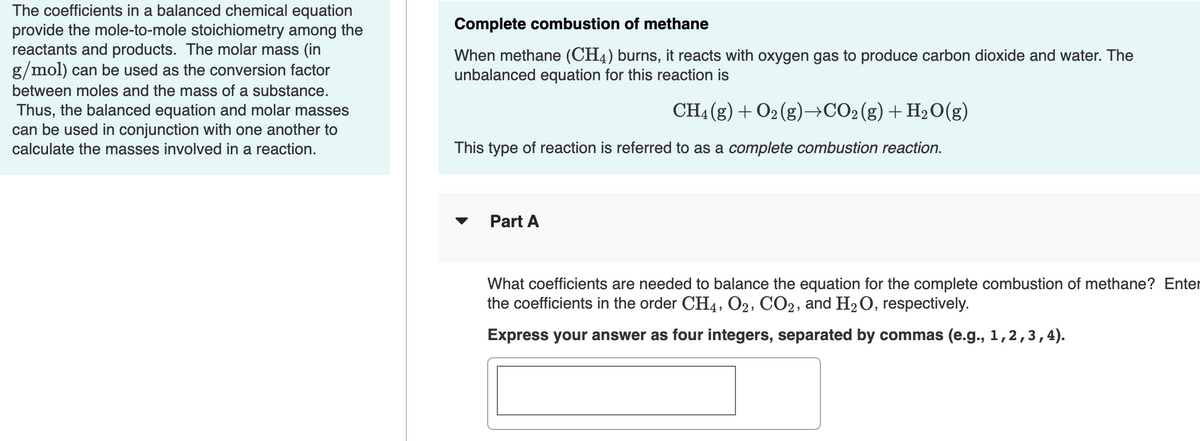
Transcribed Image Text:The coefficients in a balanced chemical equation
provide the mole-to-mole stoichiometry among the
reactants and products. The molar mass (in
g/mol) can be used as the conversion factor
Complete combustion of methane
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The
unbalanced equation for this reaction is
between moles and the mass of a substance.
Thus, the balanced equation and molar masses
can be used in conjunction with one another to
CH4 (g) + O2 (g)→CO2(g)+H2O(g)
calculate the masses involved in a reaction.
This type of reaction is referred to as a complete combustion reaction.
Part A
What coefficients are needed to balance the equation for the complete combustion of methane? Enter
the coefficients in the order CH4, O2, CO2, and H2O, respectively.
Express your answer as four integers, separated by commas (e.g., 1,2,3,4).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div